Bioactive Secondary Metabolites of a Marine Bacillus sp. Inhibit Superoxide Generation and Elastase Release in Human Neutrophils by Blocking Formyl Peptide Receptor 1
Abstract
:1. Introduction
2. Results and Discussion
2.1. IA Inhibits FMLP-Induced Superoxide Generation and Elastase Release in Human Neutrophils

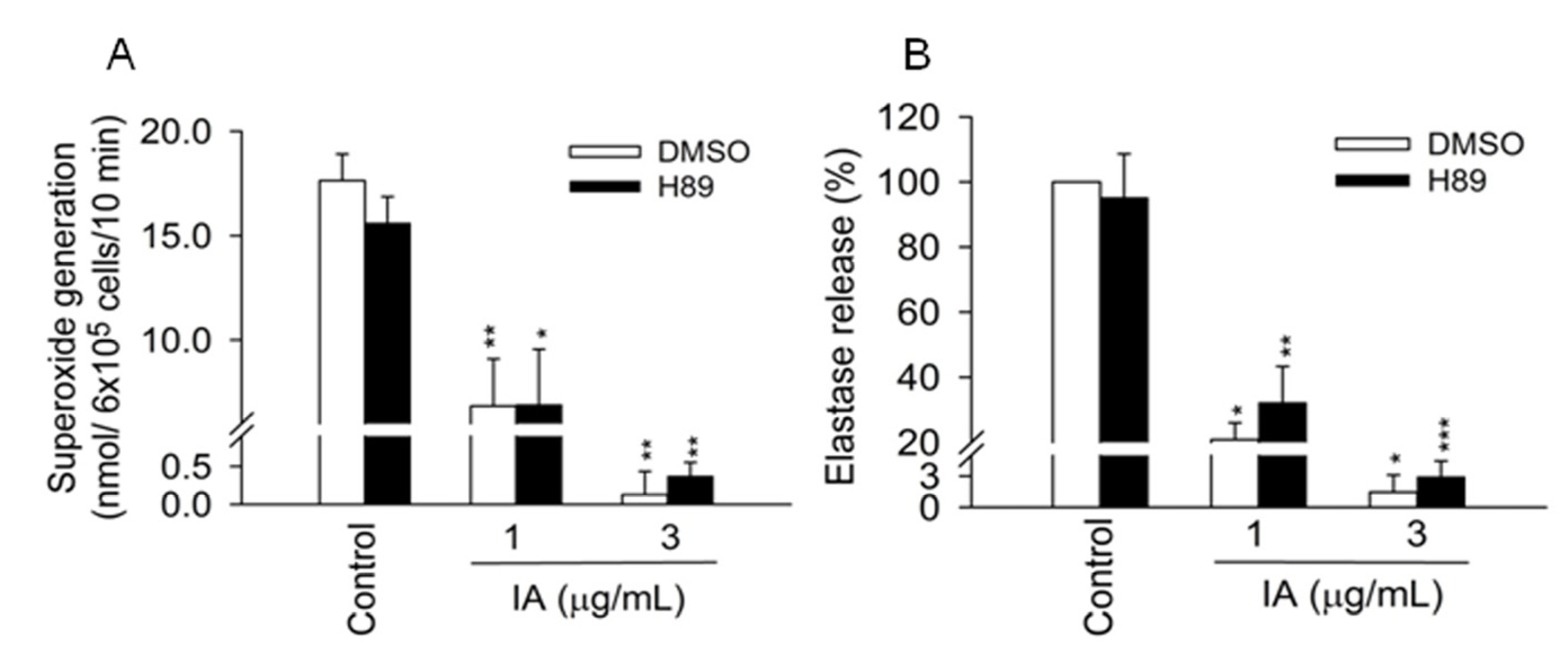
2.2. IA Does not Show Inhibition in Cell-Free Systems
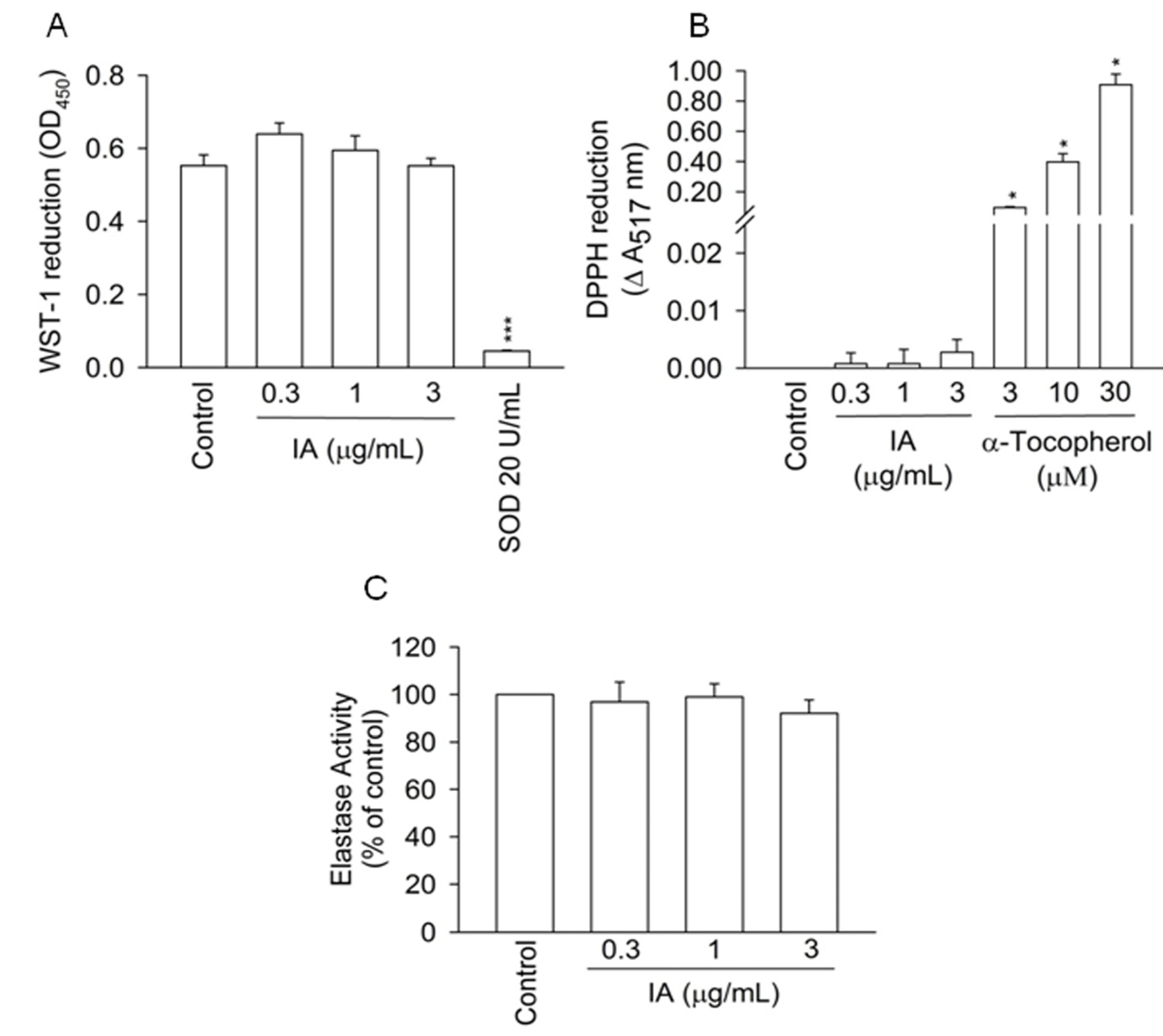
2.3. Protein Kinase A (PKA) Pathway Does not Mediate the Inhibitory Effects of IA
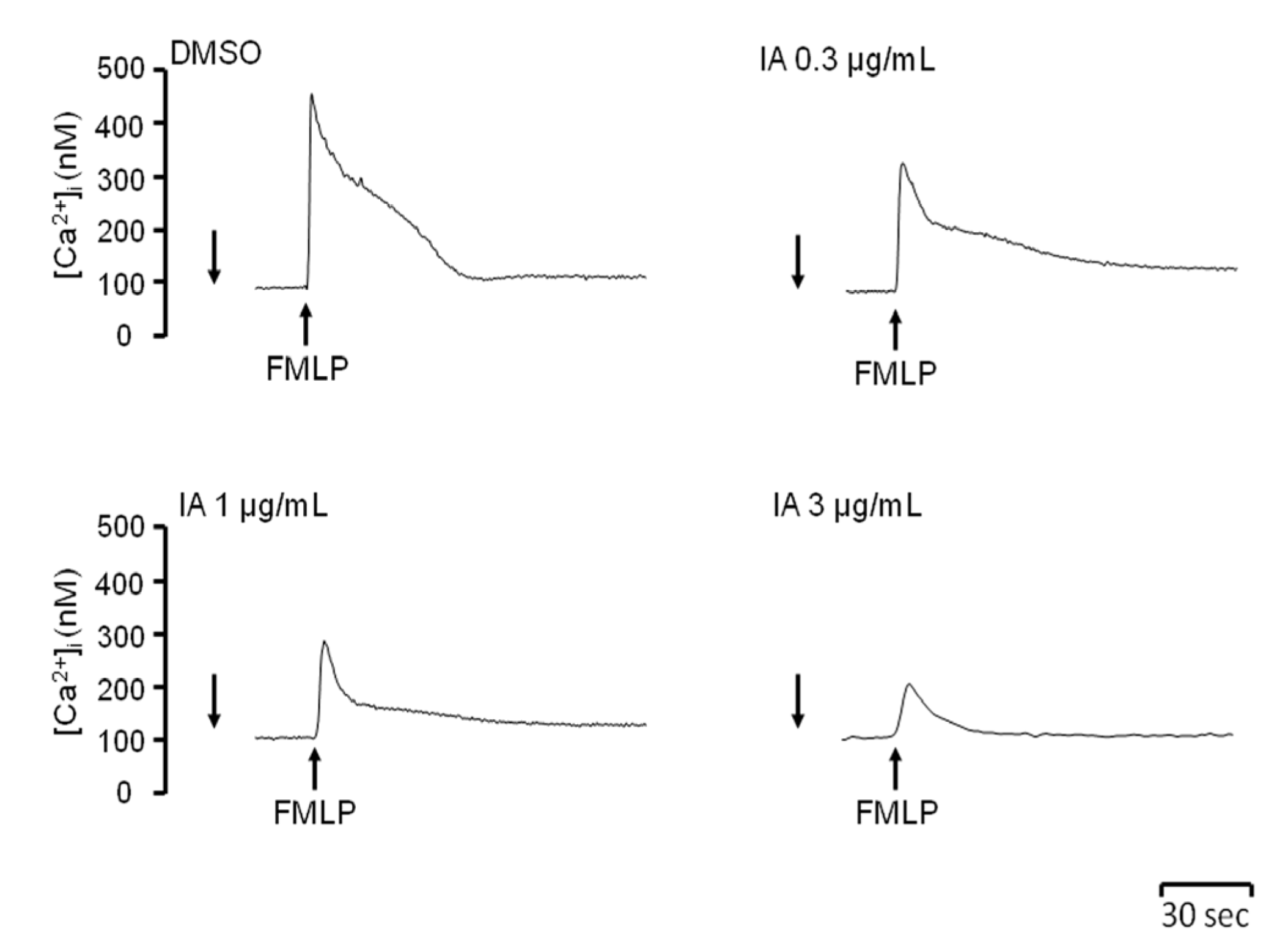
2.4. IA Attenuates Ca2+ Mobilization Induced by FMLP
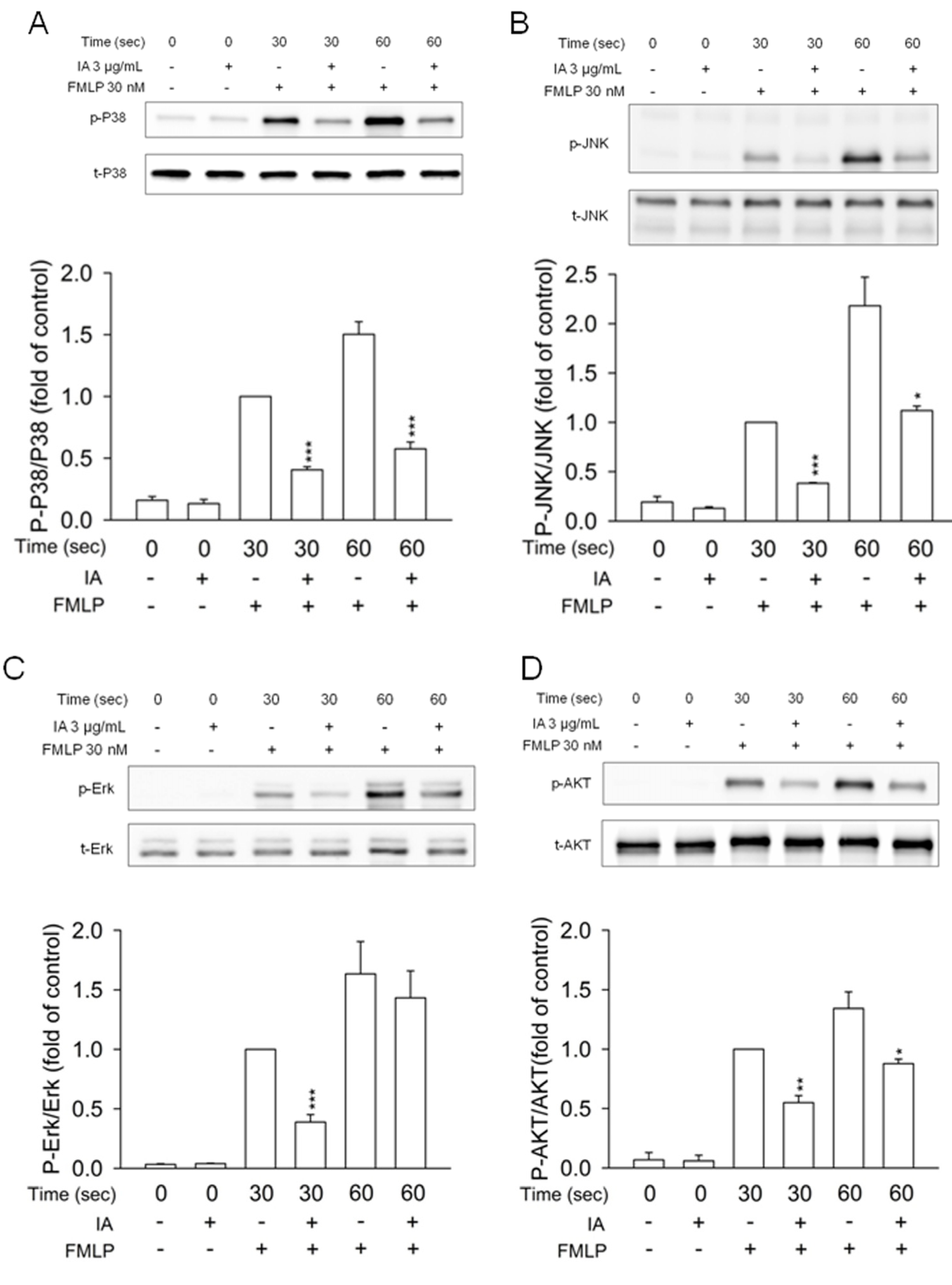
2.5. IA Inhibits Mitogen-Activated Protein (MAP) Kinases and AKT Phosphorylation in FMLP-Activated Neutrophils
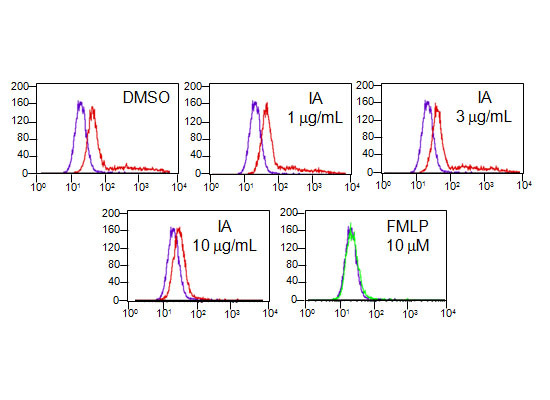
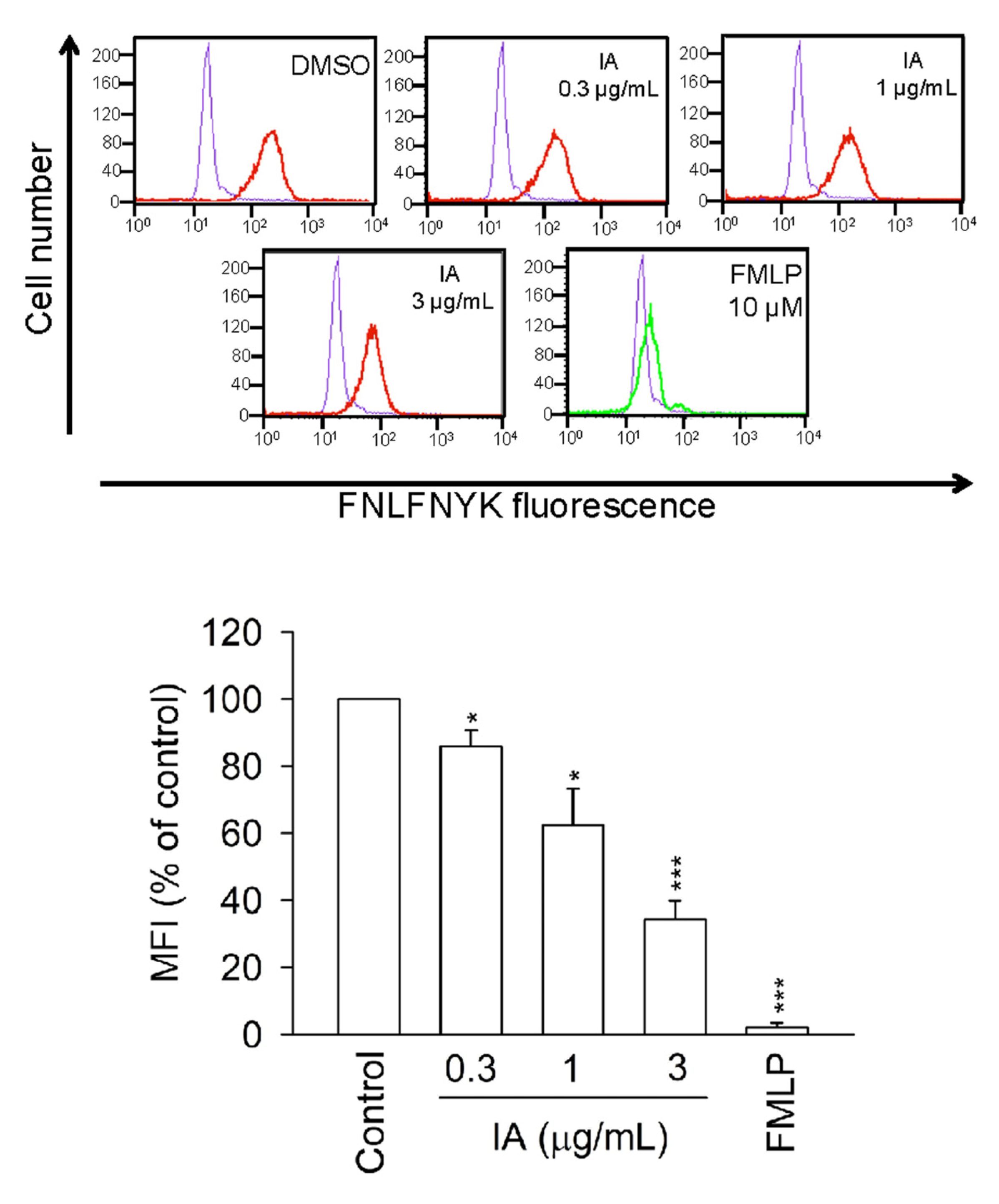
2.6. IA Inhibits the Binding of N-Formyl-Nle-Leu-Phe-Nle-Tyr-Lys-fluorescein (FNLFNYK) in Human Neutrophils
2.7. IA Inhibits the Binding of FNLFNYK in HEK293 Cells Transfected with FPR1

2.8. The High Performance Liquid Chromatography (HPLC) Method Used for Identification of the Composition of IA

3. Experimental
3.1. Reagents
3.2. Isolation of Human Neutrophils
3.3. Expression of FPR1 in Human Embryonic Kidney (HEK293) Cells
3.4. Bacterial Strains, Cultivation Condition, and Crude Extract Preparation
3.5. Measurement of Superoxide Generation and Elastase Release
3.6. Superoxide and DPPH Scavenging Assay
3.7. Evaluation of LDH Release
3.8. Receptor Binding Assay
3.9. Measurement of Intracellular Calcium Concentration ([Ca2+]i)
3.10. Immunoblotting Analysis
3.11. HPLC Fingerprint of IA
3.12. Statistics Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–70. [Google Scholar] [CrossRef]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Le, Y.; Murphy, P.M.; Wang, J.M. Formyl-peptide receptors revisited. Trends Immunol. 2002, 23, 541–548. [Google Scholar] [CrossRef]
- Carp, H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J. Exp. Med. 1982, 155, 264–275. [Google Scholar] [CrossRef]
- Marasco, W.A.; Phan, S.H.; Krutzsch, H.; Showell, H.J.; Feltner, D.E.; Nairn, R.; Becker, E.L.; Ward, P.A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984, 259, 5430–5439. [Google Scholar]
- Raoof, M.; Zhang, Q.; Itagaki, K.; Hauser, C.J. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J. Trauma 2010, 68, 1328–1332; discussion 1332–1334. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Dufton, N.; Perretti, M. Therapeutic anti-inflammatory potential of formyl-peptide receptor agonists. Pharmacol. Ther. 2010, 127, 175–188. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2010, 1790, 283–308. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar]
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef]
- Selvatici, R.; Falzarano, S.; Mollica, A.; Spisani, S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 2006, 534, 1–11. [Google Scholar] [CrossRef]
- Wu, Y.C.; Sureshbabu, M.; Fang, Y.C.; Wu, Y.H.; Lan, Y.H.; Chang, F.R.; Chang, Y.W.; Hwang, T.L. Potent inhibition of human neutrophil activations by bractelactone, a novel chalcone from Fissistigma bracteolatum. Toxicol. Appl. Pharmacol. 2013, 266, 399–407. [Google Scholar] [CrossRef]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol Inhibits Superoxide Production, Elastase Release, and Chemotaxis in Formyl Peptide-Activated Human Neutrophils by Blocking Formyl Peptide Receptor 1. J. Immunol. 2013, in press. [Google Scholar]
- Jensen, P.R.; Gontang, E.; Mafnas, C.; Mincer, T.J.; Fenical, W. Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ. Microbiol. 2005, 7, 1039–1048. [Google Scholar] [CrossRef]
- Hwang, T.L.; Hung, H.W.; Kao, S.H.; Teng, C.M.; Wu, C.C.; Cheng, S.J. Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol. Pharmacol. 2003, 64, 1419–1427. [Google Scholar] [CrossRef]
- Stenfeldt, A.L.; Karlsson, J.; Wenneras, C.; Bylund, J.; Fu, H.; Dahlgren, C. The non-steroidal anti-inflammatory drug piroxicam blocks ligand binding to the formyl peptide receptor but not the formyl peptide receptor like 1. Biochem. Pharmacol. 2007, 74, 1050–1056. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.L.; Wang, C.C.; Kuo, Y.H.; Huang, H.C.; Wu, Y.C.; Kuo, L.M.; Wu, Y.H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar]
- Gavins, F.N. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia-reperfusion injury? Trends Pharmacol. Sci. 2010, 31, 266–276. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, S.-C.; Lin, C.-F.; Chang, W.-Y.; Kuo, J.; Huang, Y.-T.; Chung, P.-J.; Hwang, T.-L. Bioactive Secondary Metabolites of a Marine Bacillus sp. Inhibit Superoxide Generation and Elastase Release in Human Neutrophils by Blocking Formyl Peptide Receptor 1. Molecules 2013, 18, 6455-6468. https://doi.org/10.3390/molecules18066455
Yang S-C, Lin C-F, Chang W-Y, Kuo J, Huang Y-T, Chung P-J, Hwang T-L. Bioactive Secondary Metabolites of a Marine Bacillus sp. Inhibit Superoxide Generation and Elastase Release in Human Neutrophils by Blocking Formyl Peptide Receptor 1. Molecules. 2013; 18(6):6455-6468. https://doi.org/10.3390/molecules18066455
Chicago/Turabian StyleYang, Shun-Chin, Chwan-Fwu Lin, Wen-Yi Chang, Jimmy Kuo, Yin-Ting Huang, Pei-Jen Chung, and Tsong-Long Hwang. 2013. "Bioactive Secondary Metabolites of a Marine Bacillus sp. Inhibit Superoxide Generation and Elastase Release in Human Neutrophils by Blocking Formyl Peptide Receptor 1" Molecules 18, no. 6: 6455-6468. https://doi.org/10.3390/molecules18066455