Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin
Abstract
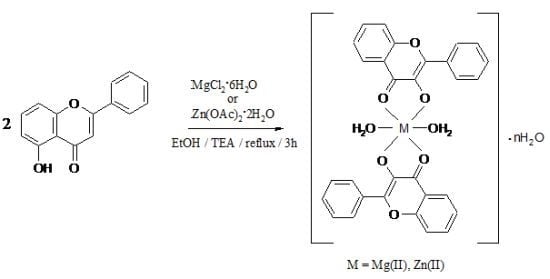
:1. Introduction

2. Results and Discussion

| Compound | DMSO | DMF | AcCN | CHCl3 | MeOH | EtOH |
|---|---|---|---|---|---|---|
| HL | soluble | soluble | soluble | soluble | soluble at mild heating | soluble at mild heating |
| [Mg(L)2(H2O)2]·H2O (1) | soluble | slightly soluble | slightly soluble | soluble at mild heating | slightly soluble | slightly soluble |
| [Zn(L)2(H2O)2]·0.5H2O (2) | soluble | soluble | soluble | soluble | slightly soluble | slightly soluble |
| Compound | Molecular formula | Molecular weight
(g mol−1) | Anal. found (calcd.) (%) | Molar conductance ΛM (Ω−1 cm2 mol−1) | ||
|---|---|---|---|---|---|---|
| C | H | M | ||||
| [Mg(L)2(H2O)2]·H2O
(1) | MgC30H24O9 | 552.82 | 64.95 (65.18) | 4.24 (4.38) | 4.10 (4.39) | 2 |
| [Zn(L)2(H2O)2]·0.5H2O
(2) | ZnC30H23O8.5 | 584.90 | 61.40 (61.60) | 4.12 (3.96) | 10.92 (11.18) | 3.5 |
2.1. IR Spectra
| Compound | ν(O-H) | ν(C=O) | ν(C=C) | ν(C-O) + δ(OH) | ν(C-O-C) | γw(H2O) |
|---|---|---|---|---|---|---|
| HL | 3,200–2,600 b, m | 1,654 s; 1,615 s | 1,587 s | 1,357 m; 1,298 s | 1,255 s | - |
| [Mg(L)2(H2O)2]·H2O (1) | 3,600–2,600 b, m | 1,634 s | 1,583 s | 1,361 m; 1,297 w | 1,251 s | 422 w |
| [Zn(L)2(H2O)2]·0.5H2O (2) | 3,600–2,600 b, m | 1,632 s | 1,580 s | 1,355 m; 1,297 w | 1,250 s | 546 w |

2.2. UV-Vis Spectra
| Compound | λmax (nm) | |
|---|---|---|
| Band I | Band II | |
| HL | 396 | 280 |
| [Mg(L)2(H2O)2]·H2O (1) | 406.5 | 284 |
| [Zn(L)2(H2O)2]·0.5H2O (2) | 403.5 | 283 |

2.3. 1H-NMR and 13CNMR Spectra
| Compound | δ of 1H (J, Hz) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H-3 | H-6 | H-7 | H-8 | H-3′ | H-4′ | H-5′ | H-2′ | H-6′ | OH | |
| HL | 7.1 (s) | 6.8 (d, 8.3) | 7.6 (t, 8.1) | 7.2 (d, 8.1) | 7.6 (m) | 8.1 (dd, 7.8, 1.7) | 12.55 (s) | |||
| (1) | 6.8 (s) | 6.4(bd, 9.9) | 7.3 (t, 8.2) | 6.3 (bd, 8.2) | 7.6 (m) | 8.0 (dd, 8.0, 2.6) | - | |||
| (2) | 7.0 (s) | 6.5 (d, 8.4) | 7.4 (t, 8.4) | 6.7 (d, 8.4) | 7.6 (m) | 8.1 (dd, 8.2, 1.7) | - | |||
| Compound | δ of 13C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 | C-9 | C-10 | |
| HL | 164.1 | 105.7 | 183.2 | 159.8 | 111.0 | 135.9 | 107.5 | 155.9 | 110.1 |
| (1) | 171.4 | 106.2 | 180.9 | 161.1 | 116.9 | 134.9 | 96.8 | 158.2 | 113.4 |
| (2) | 171.4 | 106.0 | 183.1 | 162.1 | 117.1 | 135.4 | 99.7 | 157.78 | 112.2 |
| Compound | δ of 13C | ||||||||
| C-1′ | C-2′ | C-6′ | C-3′ | C-5′ | C-4′ | ||||
| HL | 130.53 | 126.6 | 129.2 | 132.3 | |||||
| (1) | 131.11 | 126.1 | 129.2 | 131.5 | |||||
| (2) | 130.72 | 126.3 | 129.2 | 131.9 | |||||
2.4. Mass Spectra

| MLn species | Protonated molecular ion [MLn+H]+ (m/z) | Fragment at 5 eV and 1.5 mTorr argon (m/z) | Type of fragment | Fragmentation |
|---|---|---|---|---|
| 24MgL2 | 499 | 261 | 24MgL+ | [24MgL+H-L]+ |
| 25MgL2 | 500 | 262 | 25MgL+ | [25MgL+H-L]+ |
| 26MgL2 | 501 | 263 | 26MgL+ | [26MgL+H-L]+ |
| 64ZnL2 | 539 | 301 | 64ZnL+ | [64ZnL+H-L]+ |
| 66ZnL2 | 541 | 303 | 66ZnL+ | [66ZnL+H-L]+ |
| 68ZnL2 | 543 | 305 | 68ZnL+ | [68ZnL+H-L]+ |
2.5. Thermal Behavior
| Complex | Step | Thermal effect | Temperature range/°C | Δmexp/% | Δmcalc/% | Chemical process |
|---|---|---|---|---|---|---|
| [Mg(L)2(H2O)2]·H2O
(1) | 1. | Endothermic | 52–90 | 3.0 | 3.3 | H2O loss |
| 2. | Endothermic | 120–160 | 3.1 | 3.3 | H2O loss | |
| 3. | Endothermic | 170–206 | 3.2 | 3.3 | H2O loss | |
| 4. | Exothermic | 408–900 | 56.3 | Partial oxidative degradation of organic part | ||
| Residue (MgO + organic residue) | 34.4 | |||||
| [Zn(L)2(H2O)2]·0.5H2O
(2) | 1. | Endothermic | 54–75 | 1.7 | 1.6 | 0.5 H2O loss |
| 2. | Endothermic | 85–128 | 6.1 | 6.2 | 2H2O loss | |
| 3. | Exothermic | 300–750 | 78.5 | 78.3 | Oxidative degradation of organic part | |
| Residue (ZnO) | 13.7 | 13.9 | ||||
2.5.1. Thermal Decomposition of [Mg(L)2(H2O)2]·H2O

2.5.2. Thermal Decomposition of [Zn(L)2(H2O)2]·0.5H2O

2.6. Fluorescent Properties


| Compound | Excitation wavelength
λexc = 400 nm | Excitation wavelength
λexc = 429 nm | ||
|---|---|---|---|---|
| Emission wavelength
λem (nm) | Relative fluorescence
intensity (a.u.) | Emission wavelength
λem (nm) | Relative fluorescence
intensity (a.u.) | |
| HL | 540 599 | 82.43 174.87 | 456 545 642 | >1000 73.23 323.29 |
| [Mg(L)2(H2O)2]·H2O (1) | 545 598 | 510.61 379.94 | 459 548 642 | 824.65 576.89 331.28 |
| [Zn(L)2(H2O)2]·0.5H2O (2) | 548 598 | 536.83 410 | 458 548 642 | 727.71 627.11 346.30 |
3. Experimental
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Valant-Vetschera, K.M.; Bhutia, T.D.; Wollenweber, E. Exudate flavonoids of Primula spp: Structural and biogenetic chemodiversity. Nat. Prod. Commun. 2009, 4, 365–370. [Google Scholar]
- Valant-Vetschera, K.M.; Bhutia, T.D.; Wollenweber, E. Chemodiversity of exudate flavonoids in Dionysia (Primulaceae): A comparative study. Phytochemistry 2010, 71, 937–947. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr. Med. Chem. 2010, 17, 74–95. [Google Scholar] [CrossRef]
- Amić, D.; Lučić, B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg. Med. Chem. 2010, 18, 28–35. [Google Scholar] [CrossRef]
- Herath, W.; Mikell, J.R.; Hale, A.L.; Ferreira, D.; Khan, I.A. Microbial metabolism part 9. Structure and antioxidant significance of the metabolites of 5,7-dihydroxyflavone (chrysin), and 5- and 6-hydroxyflavones. Chem. Pharm. Bull. 2008, 56, 418–422. [Google Scholar] [CrossRef]
- Farkas, O.; Jakus, J.; Héberger, K. Quantitative structure-antioxidant activity relationships of flavonoid compounds. Molecules 2004, 9, 1079–1088. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Woo, Y.; Lim, Y. Relationships between structure and anti-oxidative effects of hydroxyflavones. Bull. Korean Chem. Soc. 2009, 30, 1397–1400. [Google Scholar] [CrossRef]
- Tokalov, S.V.; Kind, B.; Wollenweber, E.; Gutzeit, H.O. Biological effects of epicuticular flavonoids from Primula denticulata on human leukemia cells. J. Agric. Food. Chem. 2004, 52, 239–245. [Google Scholar] [CrossRef]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Tokalov, S.V.; Rusak, G.; Henker, Y.; Gutzeit, H.O. Biological activity of flavonoids correlate with the degree of hydroxylation. J. Appl. Biomed. 2003, 1, S33. [Google Scholar]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Ishimoto, Y.; Hotta, Y.; Hosoda, A.; Yoshikawa, H.; Akamatsu, M.; Tamura, H. Effect of flavonoids on androgen and glucocorticoid receptors based on in vitro reporter gene assay. Bioorg. Med. Chem. Lett. 2009, 19, 4706–4710. [Google Scholar] [CrossRef]
- Lättig, J.; Böhl, M.; Fischer, P.; Tischer, S.; Tietböhl, C.; Menschikowski, M.; Gutzeit, H.O.; Metz, P.; Pisabarro, M.T. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. J. Comput. Aided Mol. Des. 2007, 21, 473–483. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the structures of flavonoids and their NF-κB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011, 15, 6036–6041. [Google Scholar]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Olszanecki, R.; Gebska, A.; Kozlovski, V.I.; Gryglewski, R.J. Flavonoids and nitric oxide synthase. J. Physiol. Pharmacol. 2002, 53, 571–584. [Google Scholar]
- Shimada, T.; Tanaka, K.; Takenaka, S.; Murayama, N.; Martin, M.V.; Foroozesh, M.K.; Yamazaki, H.; Guengerich, F.P.; Komori, M. Structure-function relationships of inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 flavonoid derivatives. Chem. Res. Toxicol. 2010, 23, 1921–1935. [Google Scholar] [CrossRef]
- Cornard, J.P.; Merlin, J.C. Structural and spectroscopic investigation of 5-hydroxyflavone and its complex with aluminium. J. Mol. Struct. 2001, 569, 129–138. [Google Scholar] [CrossRef]
- Dangleterre, L.; Cornard, J.P.; Lapouge, C. Spectroscopic and theoretical investigation of the solvent effects on Al(III)-hydroxyflavone complexes. Polyhedron 2008, 27, 1581–1590. [Google Scholar] [CrossRef]
- Dangleterre, L.; Cornard, J.P. Interaction of lead (II) chloride with hydroxyflavones in methanol: A spectroscopic study. Polyhedron 2005, 24, 1593–1598. [Google Scholar] [CrossRef]
- Lapouge, C.; Dangleterre, L.; Cornard, J.P. Spectroscopic and theoretical studies of the Zn(II) chelation with hydroxyflavones. J. Phys. Chem. A 2006, 110, 12494–12500. [Google Scholar] [CrossRef]
- Hiraki, K.; Onishi, M.; Ikeda, T.; Tomioka, K.; Obayashi, Y. Syntheses of 5-hydroxyflavone-transition metal complexes. Bull. Chem. Soc. Jpn. 1978, 51, 2425–2426. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Uivarosi, V.; Marinescu, D.; Aldea, V.; Barbuceanu, S.F.; Nitulescu, G.M. Thermal behavior of some vanadyl complexes with flavone derivatives as potential insulin-mimetic agents. J. Therm. Anal. Calorim. 2011, 105, 559–564. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Cavaleiro, J.A.S.; Tarrago, G.; Marzin, C. Synthesis and characterization of ruthenium(II) complexes of 5-hydroxyflavones. J. Heterocycl. Chem. 1994, 31, 97–103. [Google Scholar] [CrossRef]
- Sai Sathish, R.; Raju Goutam, A.; Nageswara Rao, G.; Janardhana, C. A fluorescent fluoride ion probe based on a self-organized ensemble of 5-hydroxyflavone-Al(III) complex. Spectrochim. Acta A 2008, 69, 282–285. [Google Scholar] [CrossRef]
- Yuji, H.; Sano, T.; Fujii, H.; Nishio, Y.; Takanashi, H.; Hisakazu Shibata, K. Organic light-emitting diodes using 3- or 5-hydroxyflavone–metal complexes. Appl. Phys. Lett. 1997, 71, 3338–3340. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Iyyam Pillai, S.; Subramanian, S.P. Design, synthesis and characterization of zinc-3 hydroxy flavone, a novel zinc metallo complex for the treatment of experimental diabetes in rats. Eur. J. Pharmacol. 2012, 680, 122–129. [Google Scholar] [CrossRef]
- Iyyam Pillai, S.; Subramanian, S.P.; Kandaswamy, M.A. Novel insulin mimetic vanadium flavonol complex: synthesis, characterization and in vivo evaluation in STZ-induced rats. Eur. J. Med. Chem. 2013, 63, 109–117. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Batista de Carvalho, L.A.E.; Otero, J.C.; Marques, M.P.M. A conformational study of hydroxyflavones by vibrational spectroscopy coupled to DFT calculations. Spectrochim. Acta A 2013, 109, 116–124. [Google Scholar] [CrossRef]
- Kemp, W. Organic Spectroscopy, 3rd ed.; McMillan: London, UK, 1991; p. 66. [Google Scholar]
- Nakamoto, K. Infrared and Raman spectra of Inorganic Coordination Compounds, 4th ed.; John Wiley and Sons: New York, NY, USA, 2006; pp. 228–229. [Google Scholar]
- Malesev, D.; Kuntic, V. Investigation of metal-flavonoid chelates and determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.-H.; Lee, E.; Lee, Y.; Yoon, Y.; Ahn, J.-H.; Lim, Y. 1H and 13C-NMR data of hydroxyflavone derivatives. Magn. Reson. Chem. 2007, 45, 674–679. [Google Scholar] [CrossRef]
- Burns, D.C.; Ellis, D.A.; March, R.E. A predictive tool for assessing 13C-NMR chemical shifts of flavonoids. Magn. Reson. Chem. 2007, 45, 835–845. [Google Scholar] [CrossRef]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef]
- Farina, Y.; Rice, D.A. Synthesis and characterization of cobalt(II) complexes of 3-hydroxyflavone. Pertanika J. Sci. Technol. 1995, 3, 211–219. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Uivarosi, V.; Badea, M.; Olar, R.; Drǎghici, C.; Bǎrbuceanu, Ş.F. Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin. Molecules 2013, 18, 7631-7645. https://doi.org/10.3390/molecules18077631
Uivarosi V, Badea M, Olar R, Drǎghici C, Bǎrbuceanu ŞF. Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin. Molecules. 2013; 18(7):7631-7645. https://doi.org/10.3390/molecules18077631
Chicago/Turabian StyleUivarosi, Valentina, Mihaela Badea, Rodica Olar, Constantin Drǎghici, and Ştefania Felicia Bǎrbuceanu. 2013. "Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin" Molecules 18, no. 7: 7631-7645. https://doi.org/10.3390/molecules18077631