Characterization of Protein and Peptide Binding to Nanogels Formed by Differently Charged Chitosan Derivatives
Abstract
:1. Introduction
2. Results and Discussion
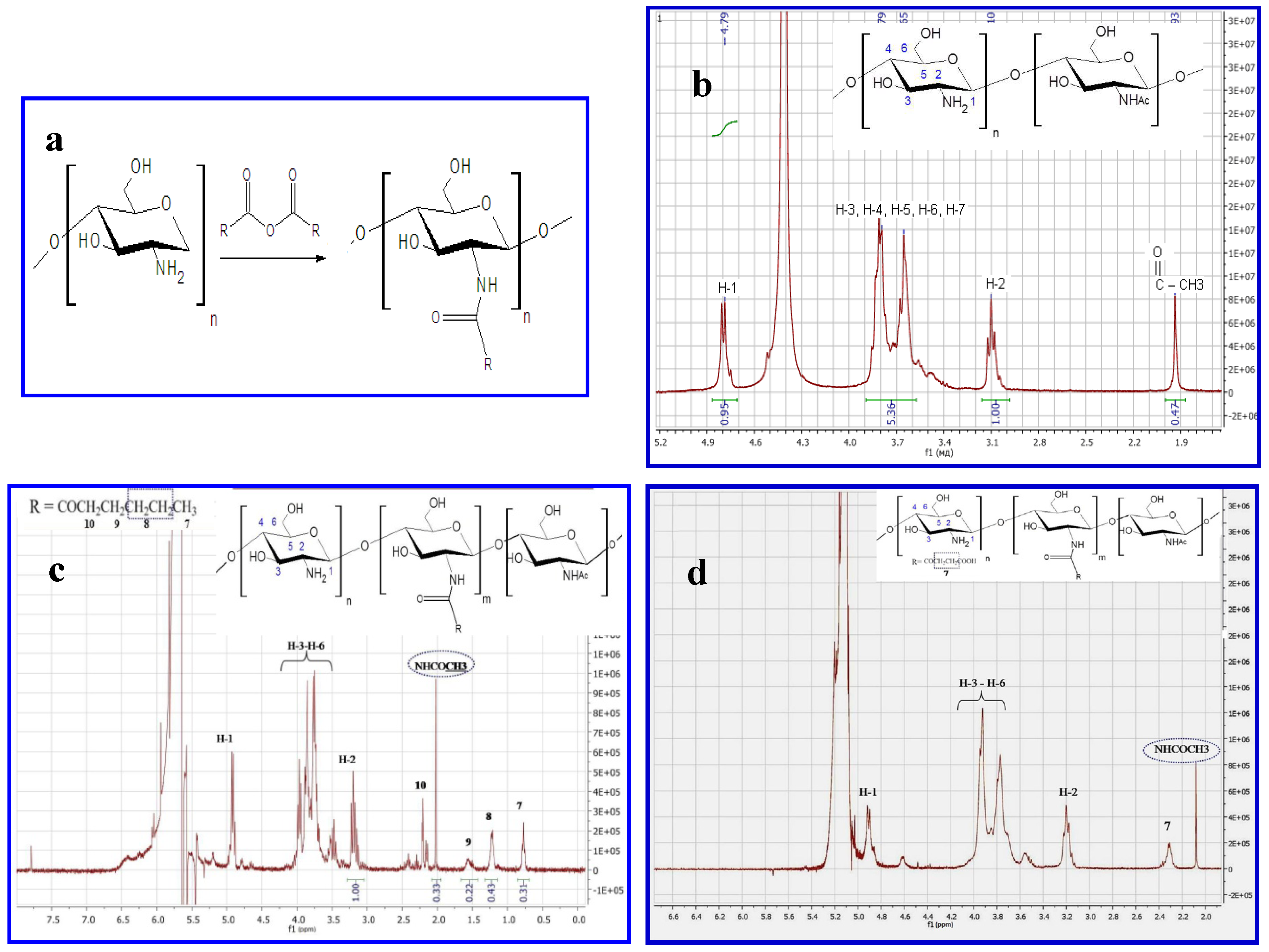
2.1. Production and Characterization of Chitosan NGs Formed by Chitosan-N-acyl Derivatives

2.2. Formation of Complexes between Chitosan NGs and Differently Charged Peptides
| Peptide | Sequences * | Charge | Sorption, % | |
|---|---|---|---|---|
| HCNGs | SCNGs | |||
| P1 | GWVDHFADGYDEVIA ** | −4 | 3.8 ± 0.8 | 0 |
| P2 | MELPSFGVSGVNESADM | −3 | 3.7 ± 0.6 | 0 |
| P3 | DQGHDTGSASAPAST | −2 | 1.4 ± 0.2 | 0 |
| P4 | VGIDQPPFGIFV | −1 | 0.8 ± 0.1 | 0 |
| P5 | PSNCHTHEGGQLHCT | −1 | 0 | 0 |
| P6 | CREGEDNSKRN | 0 | 0 | 0 |
| P7 | RMQFSSLTVNVRGSGMR | +3 | 0 | 3.8 ± 0.9 |
| P8 | KHGTFGPVHFRNQVKIR | +3 | 0 | 7.0 ± 1.1 |
2.3. Formation of Complexes between Chitosan NGs and Differently Charged Proteins
| Proteins a | Pi b | Charge c | ζ d, [mV] | MM e, [kDa] | Hydrophobicity f |
|---|---|---|---|---|---|
| BSA | 4.7 | −14 | −28 | 66 | 30 |
| Af2 | 5.3 | −16 | −28 | 37 | 33 |
| Ins | 5.4 | −4 | −9 | 5 | 39 |
| SWM | 7.6 | +2 | +5 | 17 | 37 |
| Lys | > 11 | +14 | +12 | 14 | 31 |
| Protein (ζ, [mV]) pH = 5.5–6.0 | ζ, [mV] | Sorption, % | ||
|---|---|---|---|---|
| HCNGs | SCNGs | HCNGs | SCNGs | |
| Bi-distilled water (0 ± 2) | +27 ± 5 | −27 ± 5 | ||
| BSA (−15 ± 5) | +14 ± 2 * | −23 ± 5 | 46 ± 5 | 47 ± 8 ** |
| Af2 (−16 ± 5) | +15 ± 2 * | −21 ± 2 | 23 ± 3 | 0 ± 3 |
| Ins (−9 ± 3) | +9 ± 3 * | −21 ± 3 | 42 ± 7 | 0 ± 3 |
| SWM (+5 ± 4) | +26 ± 5 | −16 ± 3 * | 10 ± 2 | 36 ± 5 |
| Lys (+12 ± 2) | +26 ± 5 | 0 ± 2 * | 6 ± 3 | 43 ± 2 |
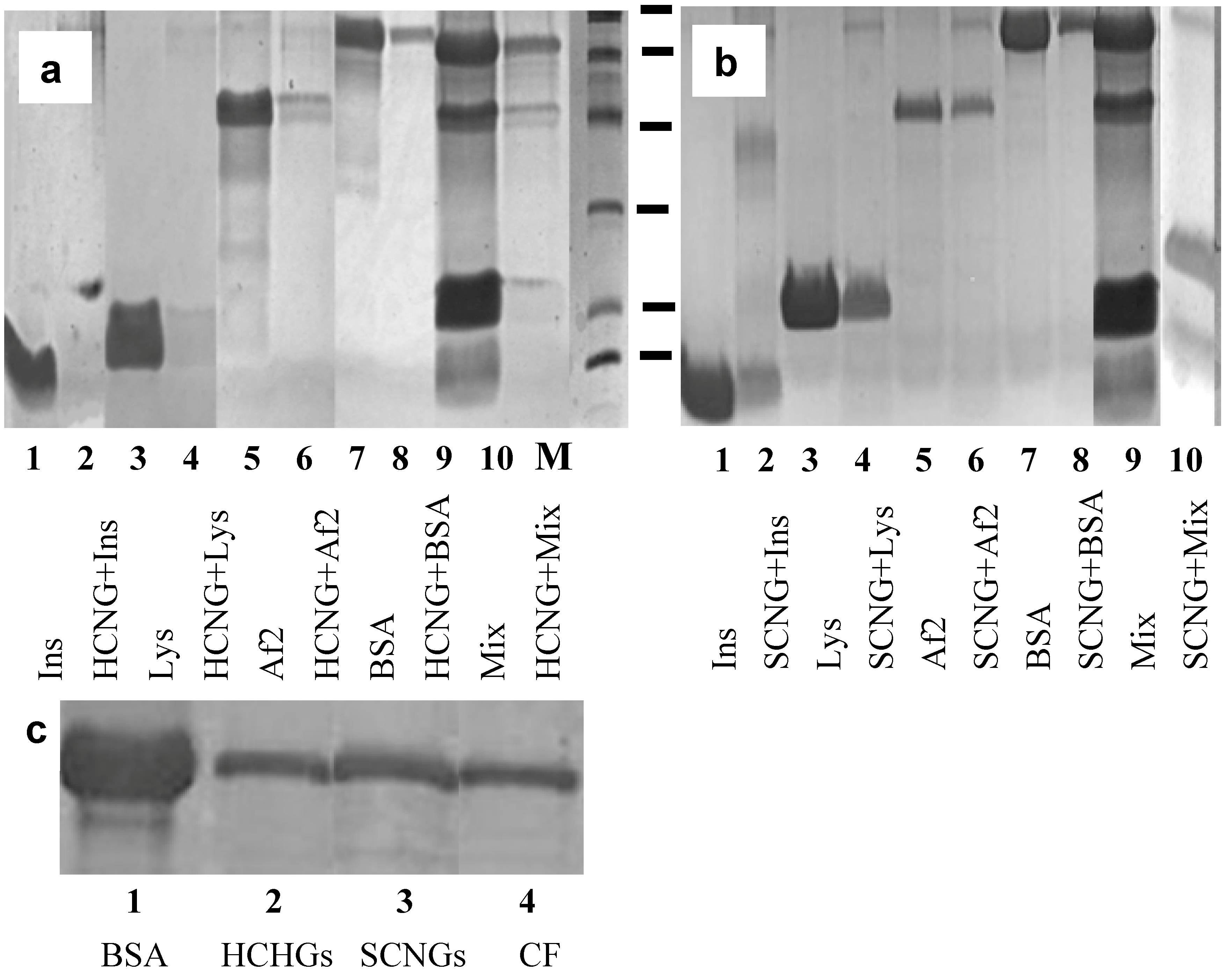
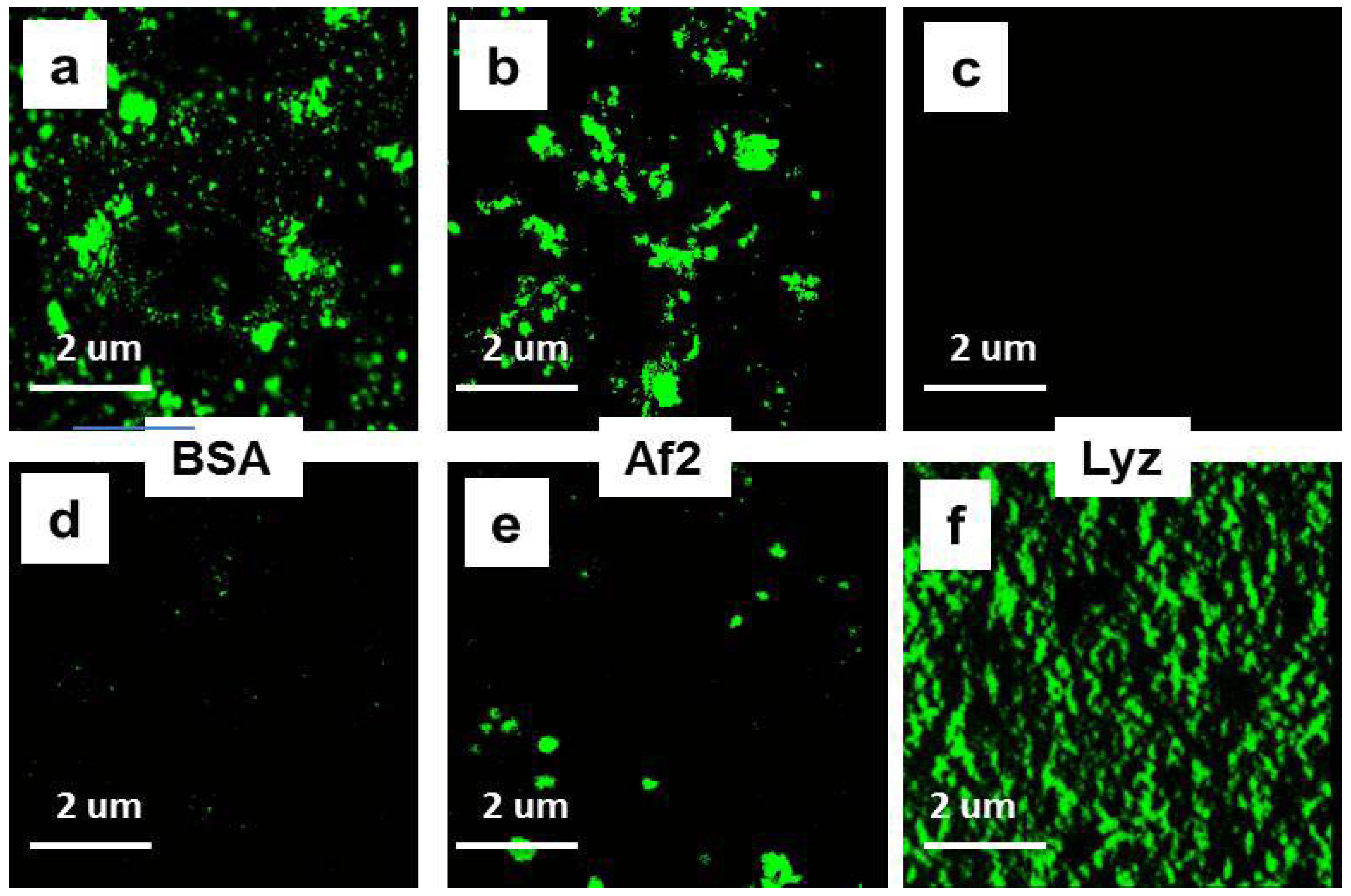
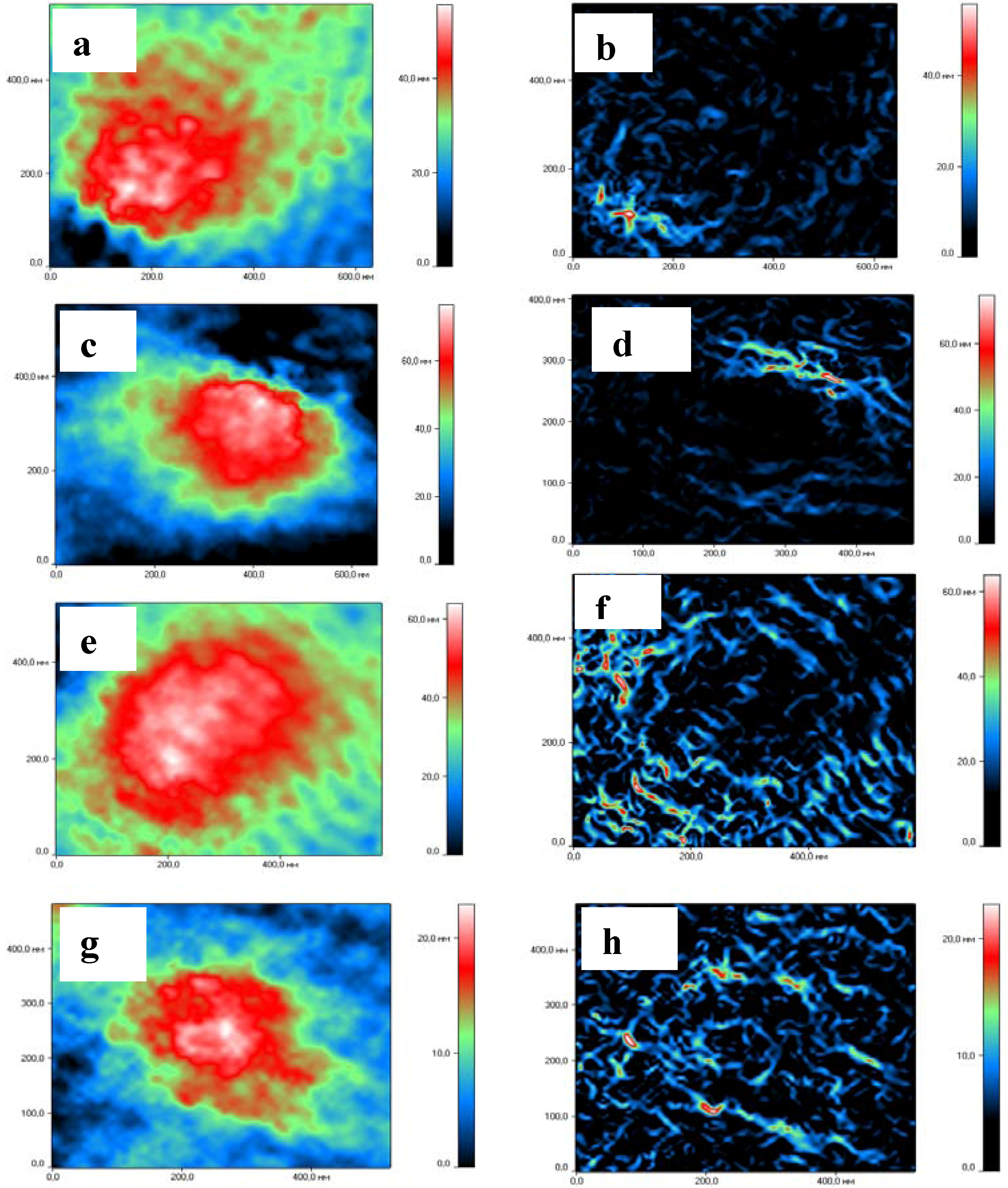
2.4. Interaction of Chitosan NGs with Plasma Proteins
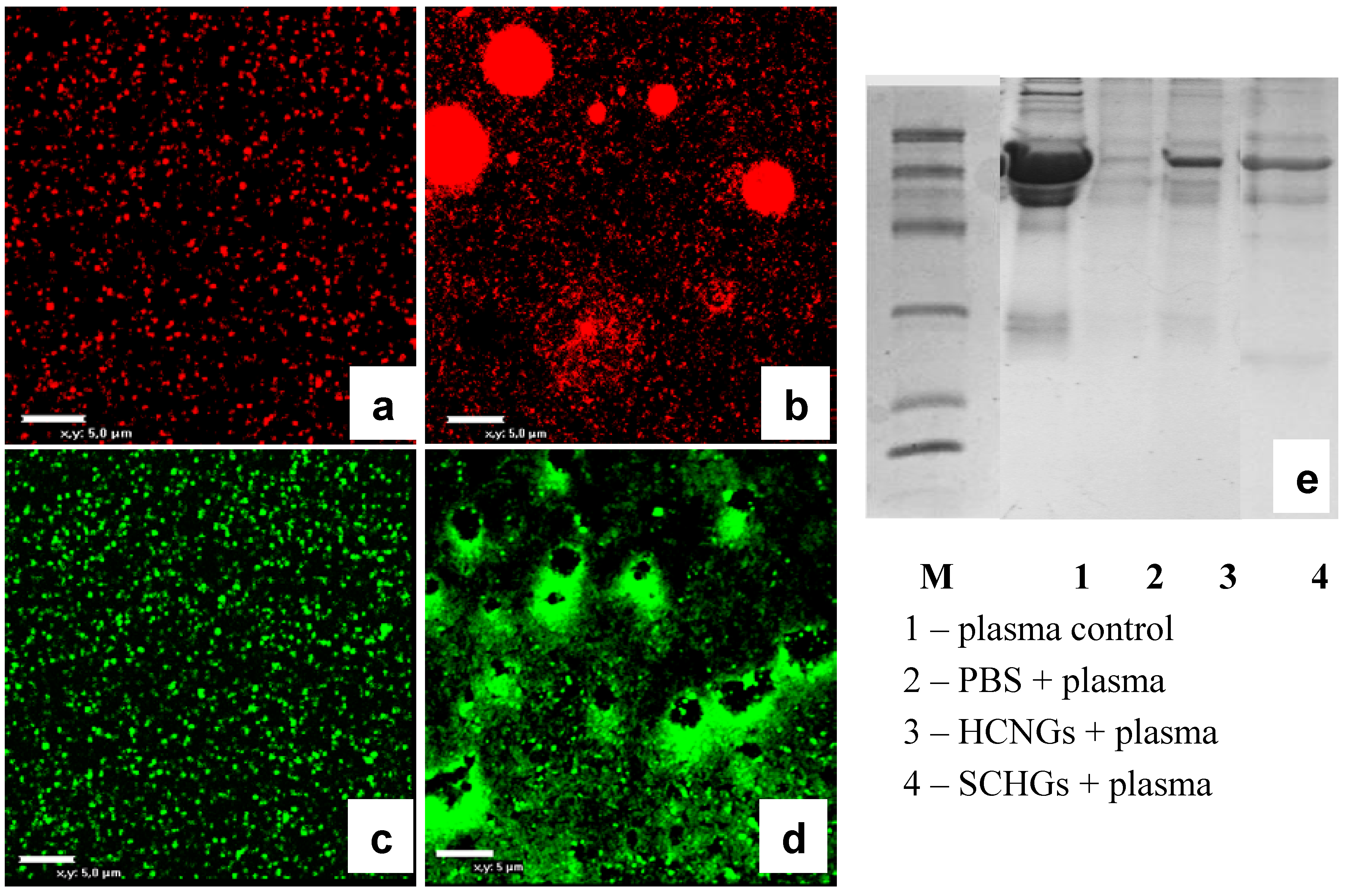
3. Experimental
3.1. Materials
3.2. Preparations of Chitosan Nanogels
3.2.1. N-hexanoylchitosan Nanogels (HCNGs)
3.2.2. Succinoylchitosan Nanogels (SCNGs)
3.3. Characterization of Chitosan Nanogels
3.3.1. Dynamic Light Scattering (DLS)
3.3.2. Atomic Force Microscopy (AFM)
3.3.3. Nanoparticle Tracking Analysis (NTA)
3.3.4. Laser Interference Microscopy
3.4. Complex Formation between Chitosan Nanogels and Various Peptides
3.4.1. Complex Formation between Chitosan Nanogels and Various Proteins
3.4.2. Visualization of NGs
3.4.3. Chitosan NG Binding to Blood Plasma Proteins
3.5. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Janes, K.A.; Fresneau, M.P.; Marazuela, A; Fabra, A; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Garg, N.K.; Dwivedi, P.; Campbell, C.; Tyagi, R.K. Site specific/targeted delivery of gemcitabine through anisamide anchored chitosan/poly ethylene glycol nanoparticles: An improved understanding of lung cancer therapeutic intervention. Eur. J. Pharm. Sci. 2012, 47, 1006–1014. [Google Scholar] [CrossRef]
- Jin, Y.H.; Hu, H.Y,; Qiao, M.X.; Zhu, J.; Qi, J.W.; Hu, C.J.; Zhang, Q.; Chen, D.W. pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: Preparation and in vitro evaluation. Colloids Surf. B 2012, 94, 184–191. [Google Scholar]
- Fernandez-Urrusuno, R.; Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef]
- Lebre, F.; Bento, D.; Jesus, S.; Borges, O. Chitosan-based nanoparticles as a hepatitis B antigen delivery system. Methods Enzymol. 2012, 509, 127–142. [Google Scholar] [CrossRef]
- Trapani, A.; Lopedota, A.; Franco, M.; Cioffi, N.; Ieva, E.; Garcia-Fuentes, M.; Alonso, M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur. J. Pharm. Biopharm. 2010, 75, 26–32. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of highmolecular weight kininogen, factor XII, andfibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar]
- Chen, F.; Zhang, Z.R.; Huang, Y. Evaluation and modification of N-trimethyl chitosan chloride nanoparticles as protein carriers. Int. J. Pharm. 2007, 336, 166–173. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.G.; Huang, L.; Han, J.T.; Zhang, X.F. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: Biocompatibility, protein adsorption and cellular uptake. J. Mater. Sci. Mater. Med. 2012, 23, 1775–1783. [Google Scholar]
- Gan, Q.; Wang, T. Chitosan nanoparticles as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Zhu, Ai-ping; Yuan, Lan-hua; Chen, Tian; Wu, Hao; Zhao, Feng. Interactions between N-succinyl-chitosan and bovine serum albumin. Carbohydr. Polym. 2007, 69, 363–370. [Google Scholar]
- Graisuwan, W.; Wiarachai, O.; Ananthanawat, C.; Puthong, S.; Soogarun, S.; Kiatkamjornwong, S.; Hoven, V.P. Multilayer film assembled from charged derivatives of chitosan: Physical characteristics and biological responses. J. Colloid Interface Sci. 2012, 376, 177–188. [Google Scholar] [CrossRef]
- Celik, O.; Akbuğa, J. Preparation of superoxide dismutase loaded chitosan microspheres: characterization and release studies. Eur. J. Pharm. Biopharm. 2007, 66, 42–47. [Google Scholar] [CrossRef]
- Ma, Z.; Yeoh, H.H.; Lim, L.-Y. Formulation pH modulates the interaction of insulin with chitosan nanoparticles. J. Control. Release 2000, 37, 124–132. [Google Scholar]
- Wang, S.; Jiang, T.; Ma, M.; Hu, Y.; Zhang, J. Preparation and evaluation of anti-neuroexcitation peptide (ANEP) loaded N-trimethyl chitosan chloride nanoparticles for brain-targeting. Int. J. Pharm. 2010, 386, 249–255. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Park, K.; Kang, E.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Chi, D.Y.; Park, R.W.; et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 2008, 29, 1920–1930. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, S.; Nam, J.O.; Park, R.W.; Chung, H.; Seo, S.B.; Kim, I.S.; Kwon, I.C.; Jeong, S.Y. Self-assembled nanoparticles based on glycol chitosan bearing 5h-cholanic acid for RGD peptide delivery. J. Control. Release 2004, 95, 579–588. [Google Scholar] [CrossRef]
- Zou, X.; Zhong, L.; Liu, D.; Yang, B.; Lou, Y.; Peng, J.; Rainer, M.; Feuerstein, I.; Muhammad, N.U.; Huck, C.W.; et al. Novel multifunctional chitosan-GMA-IDA-Cu(II) nanospheres for high dynamic range characterization of the human plasma proteome. Anal. Bioanal. Chem. 2011, 400, 747–756. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H.J. Preparation, characterization and protein loading of hexanoyl-modified chitosan nanoparticles. Drug Deliv. 2006, 13, 375–381. [Google Scholar] [CrossRef]
- Il’ina, A.V.; Zubareva, A.A.; Kurek, D.V.; Levov, A.N; Varlamov, V.P. Nanoparticles Based on Succinylchitosan with Doxorubicin: Preparation and Properties. Nanotechnol. Russ. 2012, 7, 85–92. [Google Scholar]
- Rekha, M.R.; Sharma, C.P. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J. Control. Release 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Sahu, S.K.; Maiti, S.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Folate-decorated succinylchitosan nanoparticles conjugated with doxorubicin for targeted drug delivery. Macromol. Biosci. 2011, 11, 285–295. [Google Scholar] [CrossRef]
- Yan, C.; Gu, J.; Guo, Y.; Chen, D. In vivo biodistribution for tumor targeting of 5-fluorouracil (5-FU) loaded N-succinyl-chitosan (Suc-Chi) nanoparticles. Yakugaku Zasshi 2010, 130, 801–804. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 293–329. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, J.C.; Seabra, V.; Sarmento, B. Chitosan-based nanoparticles as delivery systems of therapeutic proteins. Methods Mol. Biol. 2012, 899, 471–487. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef]
- Jie, L.Y.; Cai, L.L.; Wang, L.J.; Ying, X.Y.; Yu, R.S.; Zhang, M.M.; Du, Y.Z. Actively-targeted LTVSPWY peptide-modified magnetic nanoparticles for tumor imaging. Int. J. Nanomed. 2012, 7, 3981–3989. [Google Scholar]
- Lalatsa, A.; Garrett, N.L.; Ferrarelli, T.; Moger, J.; Schätzlein, A.G.; Uchegbu, I.F. Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol. Pharm. 2012, 9, 1764–1774. [Google Scholar] [CrossRef]
- Hu, C.S.; Chiang, C.H.; Hong, P.D.; Yeh, M.K. Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers. Int. J. Nanomed. 2012, 7, 4861–4872. [Google Scholar]
- Lee, J.Y.; Lee, S.H.; Oh, M.H.; Kim, J.S.; Park, T.G.; Nam, Y.S. Prolonged gene silencing by siRNA/chitosan-g-deoxycholic acid polyplexes loaded within biodegradable polymer nanoparticles. J. Control. Release 2012, 162, 407–413. [Google Scholar]
- Huang, R.; Yang, W.; Jiang, C.; Pei, Y. Gene delivery into brain capillary endothelial cells using Antp-modified DNA-loaded nanoparticles. Chem. Pharm. Bull. 2006, 54, 1254–1258. [Google Scholar] [CrossRef]
- Il’ina, A.V.; Varlamov, V.P. Effect of the degree of acetylation of chitosan on its enzymatic hydrolysis with the preparation Celloviridin G20x. Appl. Biochem. Microbiol. 2003, 39, 239–243. [Google Scholar] [CrossRef]
- Il’ina, A.V.; Varlamov, V.P.; Ermakov, Yu. A.; Orlov, V.N.; Skryabin, K. Chitosan is a Natural Polymer for Constructing Nanoparticles. Dokl. Chem. 2008, 421, 165–167. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zubareva, A.; Ilyina, A.; Prokhorov, A.; Kurek, D.; Efremov, M.; Varlamov, V.; Senel, S.; Ignatyev, P.; Svirshchevskaya, Е. Characterization of Protein and Peptide Binding to Nanogels Formed by Differently Charged Chitosan Derivatives. Molecules 2013, 18, 7848-7864. https://doi.org/10.3390/molecules18077848
Zubareva A, Ilyina A, Prokhorov A, Kurek D, Efremov M, Varlamov V, Senel S, Ignatyev P, Svirshchevskaya Е. Characterization of Protein and Peptide Binding to Nanogels Formed by Differently Charged Chitosan Derivatives. Molecules. 2013; 18(7):7848-7864. https://doi.org/10.3390/molecules18077848
Chicago/Turabian StyleZubareva, Anastasia, Alla Ilyina, Aleksander Prokhorov, Denis Kurek, Mikhail Efremov, Valery Varlamov, Sevda Senel, Pavel Ignatyev, and Еlena Svirshchevskaya. 2013. "Characterization of Protein and Peptide Binding to Nanogels Formed by Differently Charged Chitosan Derivatives" Molecules 18, no. 7: 7848-7864. https://doi.org/10.3390/molecules18077848




