Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study
Abstract
:1. Introduction
2. Results and Discussion

2.1. Susceptibility Testing
| C.a. (ATCC) | C.a. (SC5314) | C.k. | C.p. | C.t. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | CC50 |
| Extract | 156 | 625 | 156 | 156 | 19 | 39 | 78 | 156 | 312 | 1250 | 417 |
| EAF | 625 | 1250 | NT | NT | 19 | 78 | 312 | 625 | 312 | 625 | 221 |
| BF | 78 | 1250 | 156 | 156 | 19 | 39 | 19 | 19 | 156 | 1250 | 767 |
| AF | 312 | >1250 | NT | NT | 39 | 78 | 39 | 78 | 312 | 1250 | 1500 |
| F2 | 78 | 312 | 156 | 312 | 625 | >1250 | 156 | 1250 | 312 | >1250 | >400 |
| casuarinin | 580 | 580 | 580 | >580 | 26 | 580 | 580 | 580 | 145 | 580 | >116 |
| FCZ | 4 | 8 | 2 | 32 | 32 | 32 | 4 | 4 | 32 | 32 | NA |
| Amph B | 0.25 | 0.25 | 1 | 8 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 2 | NA |
2.2. Ergosterol Levels
| SAMPLES | C. albicans | C. krusei | C. parapsilosis | C. tropicalis |
|---|---|---|---|---|
| Control | 1.38 ± 0.32 | 1.13 ± 0.70 | 0.34 ± 0.30 | 1,94 ± 0.10 |
| BF | 2.03 ± 0.77(+48) | 1.44 ± 0.58(+28) | 0.77 ± 0.70(+29) | 1,30 ± 0.86(−33) |
| FCZ | 0.40 ± 0.23(−71) * | 0.68 ± 0.08(−40) | 0.05 ± 0.05(−84) * | 0(−100) * |
2.3. Reduction of P. Cauliflora Leaf Samples Antifungal Properties
2.3.1. Addition of Exogenous Ergosterol
2.3.2. Sorbitol Protection Assay
| Ergosterol | Sorbitol | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | C.a. | C.k. | C.p. | C.t. | C.a. | C.k. | C.p. | C.t. |
| Extract | 5000 | 39 | 312 | 625 | 2500 | 39 | 2500 | 156 |
| EAF | 5000 | 39 | 1250 | 625 | 5000 | 39 | 2500 | 312 |
| BF | 5000 | 39 | 1250 | 312 | 5000 | 39 | 1250 | 312 |
| AF | 5000 | 78 | 2500 | 625 | 5000 | 78 | 2500 | 1250 |
| F2 | 1250 | 78 | 1250 | 312 | 1250 | 78 | 625 | 312 |
| FCZ | NA | NA | NA | NA | 8 | 64 | 4 | 64 |
| Amph B | 2 | 16 | 16 | 4 | 0.25 | 1 | 1 | 1 |
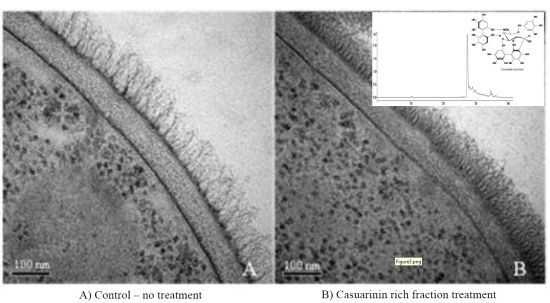
2.4. Ultrastructural Analysis

2.5. Assessing C. albicans cell wall treated with F2
| Control cells | Treated cells | |||
|---|---|---|---|---|
| Yeast | Hyphae | Yeast | Hyphae | |
| Chitin (%) | 5.1 ± 1.8 | 21.8 ± 1.3 | 4.2 ± 1.4 | 22.1 ± 4.0 |
| Glucan (%) | 66.3 ± 4.9 | 68.8 ± 0.6 | 73.4 ± 4.0 | 67.3 ± 2.0 |
| Mannan (%) | 28.6 ± 5.6 | 9.4 ± 1.6 | 22.4 ± 5.3 | 10.6 ± 2.1 |
| Covalently attached cell wall protein (µg/mL) | 109.4 ± 5.5 | 90.2 ± 5.0 | 114.5 ± 4.2 | 101.3 ± 2.0 * |
| Non-covalently attached cell wall protein (µg/mL) | 226.5 ± 1.5 | 156.6 ± 0.5 | 227.6 ± 3.4 | 173.3 ± 0.1 |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Fungal Strains, Cell Line and Culture Conditions
3.5. Antifungal Susceptibility Testing
3.6. Cytotoxicity Testing
3.7. Sterol Quantification Method
3.8. Ultrastructural Analysis by Transmission Electron Microscopy (TEM)
3.9. Cell Wall Polymer Quantification
3.10. Relative Cell Wall Porosity
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sobral, M. Alterações nomenclaturais em Plinia (Myrtaceae). Boletim do Museu Botânico de Curitiba 1985, 63, 1–4. [Google Scholar]
- Lorenzi, H. Árvores brasileiras: Manual de identificação e cultivo de plantas arbóreas nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2000. [Google Scholar]
- Agra, M.F.; Silva, K.N.; Basílio, I.J.L.D.; Freitas, P.F.; Barbosa-Filho, J.M. Survey of medicinal plants used in the region northeast of Brazil. Braz. J. Pharmacogn. 2008, 18, 472–508. [Google Scholar]
- Reynertson, K.A.; Wallace, A.M.; Adachi, S.; Gil, R.R.; Yang, H.; Basile, M.J.; D’Armiento, J.; Weinstein, I.B.; Kennelly, E.J. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora). J. Nat. Prod. 2006, 69, 1228–1230. [Google Scholar] [CrossRef]
- Souza-Moreira, T.M.; Moreira, R.R.D.; Sacramento, L.V.S.; Pietro, R.C.L.R. Histochemical, phytochemical and biological screening of Plinia cauliflora (DC.) Kausel, Myrtaceae, leaves. Braz. J. Pharmacogn. 2010, 20, 48–53. [Google Scholar]
- Calderone, R.A. Candida and Candidiasis; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Richardson, M.; Lass-Flörl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef]
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the mechanism of action of the antifungal phytolaccoside b isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef]
- Hoehamer, C.F.; Cummings, E.D.; Hilliard, G.M.; Rogers, P.D. Changes in the proteome of Candida albicans in response to azole, polyene, and echinocandin antifungal agents. Antimicrob. Agents Chemother. 2010, 54, 1655–1664. [Google Scholar] [CrossRef]
- Zhang, J.D.; Xu, Z.; Cao, Y.B.; Chen, H.S.; Yan, L.; An, M.M.; Gao, P.H.; Wang, Y.; Jia, X.M.; Jiang, Y.Y. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J. Ethnopharmacol. 2006, 103, 76–84. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Gertsch, J.; Tobler, R.T.; Brun, R.; Sticher, O.; Heilmann, J. Antifungal, antiprotozoal, cytotoxic and piscicidal properties of justicidin b and a new arylnaphthalide lignan from Phyllanthus piscatorum. Planta Med. 2003, 69, 420–424. [Google Scholar] [CrossRef]
- Ishida, K.; de Mello, J.C.P.; Cortez, D.A.G.; Filho, B.P.D.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannins of Casuarina and Stachyurus species. I: Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. I 1983, 8, 1765–1772. [Google Scholar]
- Yoshimura, M.; Ito, H.; Miyashita, K.; Hatano, T.; Taniguchi, S.; Amakura, Y.; Yoshida, T. Flavonol glucuronides and c-glucosidic ellagitannins from Melaleuca squarrosa. Phytochemistry 2008, 69, 3062–3069. [Google Scholar] [CrossRef]
- Araujo, M.G.; Hilario, F.; Nogueira, L.G.; Vilegas, W.; Santos, L.C.; Bauab, T.M. Chemical constituents of the methanolic extract of leaves of Leiothrix spiralis Ruhland and their antimicrobial activity. Molecules 2011, 16, 10479–10490. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Perrot, S.; Dutertre-Catella, H.; Martin, C.; Rat, P.; Warnet, J.-M. Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol. Sci. 2003, 72, 122–129. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.R.; Lunde, C.S.; Kubo, I. In vitro antifungal susceptibilities of Candida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Med. 1999, 65, 204–208. [Google Scholar] [CrossRef]
- De Nobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; van den Ende, H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef]
- Lee, K.; Buckley, H.; Campbell, C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 1975, 13, 148–153. [Google Scholar] [CrossRef]
- Munro, C.A.; Whitton, R.K.; Bleddyn Hughes, H.; Rella, M.; Selvaggini, S.; Gow, N.A.R. Chs8-a fourth chitin synthase gene of Candida albicans contributes to in vitro chitin synthase activity, but is dispensable for growth. Fungal Genet. Biol. 2003, 40, 146–158. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute document M27-A2. In Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002.
- Walker, L.A.; Munro, C.A.; Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A.R. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Sánchez-Medina, A.; Stevenson, P.C.; Habtemariam, S.; Peña-Rodríguez, L.M.; Corcoran, O.; Mallet, A.I.; Veitch, N.C. Triterpenoid saponins from a cytotoxic root extract of Sideroxylon foetidissimum subsp. gaumeri. Phytochemistry 2009, 70, 765–772. [Google Scholar] [CrossRef]
- Netea, M.G.; Gow, N.A.R.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and toll-like receptors. J. Clin. Invest. 2006, 116, 1642–1650. [Google Scholar]
- Groot, P.W.J.; de Boer, A.D.; Cunningham, J.; Dekker, H.L.; de Jong, L.; Hellingwerf, K.J.; de Koster, C.; Klis, F.M. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryotic. Cell 2004, 3, 955–965. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Souza-Moreira, T.M.; Severi, J.A.; Lee, K.; Preechasuth, K.; Santos, E.; Gow, N.A.R.; Munro, C.A.; Vilegas, W.; Pietro, R.C.L.R. Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study. Molecules 2013, 18, 8095-8108. https://doi.org/10.3390/molecules18078095
Souza-Moreira TM, Severi JA, Lee K, Preechasuth K, Santos E, Gow NAR, Munro CA, Vilegas W, Pietro RCLR. Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study. Molecules. 2013; 18(7):8095-8108. https://doi.org/10.3390/molecules18078095
Chicago/Turabian StyleSouza-Moreira, Tatiana M., Juliana A. Severi, Keunsook Lee, Kanya Preechasuth, Emerson Santos, Neil A. R. Gow, Carol A. Munro, Wagner Vilegas, and Rosemeire C. L. R. Pietro. 2013. "Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study" Molecules 18, no. 7: 8095-8108. https://doi.org/10.3390/molecules18078095