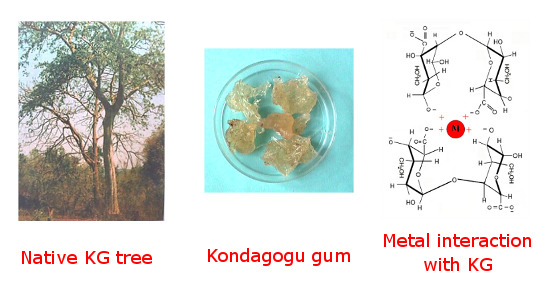
Morphology and Metal Binding Characteristics of a Natural Polymer—Kondagogu (Cochlospermum gossypium) Gum
Abstract
:1. Introduction
2. Results and discussion
2.1. SEM-EDXA Analysis of KG and Its Metal Complexes


2.2. AFM Analysis of KG and Metal Complexes

2.3. TEM Analysis

2.4. Small Angle X-Ray Scattering Analysis


3. Experimental
3.1. Materials and Methods
3.2. Metal Solutions
3.3. Biosorbent Preparation
3.4. Preparation of KG-Metal Complexes
3.5. SEM-EDXA Analysis
3.6. Atomic Force Microscopic (AFM) Analysis
3.7. Transmission Electron Microscopic (TEM) Analysis
3.8. Small Angle X-Ray Scattering (SAXS) Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- BeMiller, J.N.; Salamone, J.C. Polymeric Materials Encyclopedia; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Cottrell, I.L.; Baird, J.K.; Mark, H.F.; Other, D.F.; Berger, O.C.G.; Seaborg, G.T. Encyclopedia of Kirk-Othmer’s Chemical Technology; Wiley Interscience: New York, NY, USA, 1980. [Google Scholar]
- Verbeken, D.; Dierchx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and application. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Janaki, B.; Sashidhar, R.B. Physico-chemical analysis of gum kondagogu (Cochlospermum gossypium): A potential food additive. Food Chem. 1998, 61, 231–236. [Google Scholar] [CrossRef]
- Janaki, B.; Sashidhar, R.B. Sub-chronic (90-day) Toxicity study in rats fed gum kondagogu (Cochlospermum gossypium). Food Chem. Toxicol. 2000, 38, 523–534. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Suresh, K.I.; Rama Rao, B.; Vijaya Saradhi, U.V.R.; Prabhakar Rao, T. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sarma, V.U.M.; Vijaya Saradhi, U.V.R. Compositional analysis and rheological properties of gum kondagogu (Cochlospermum gossypium): A Tree gum from India. J. Agric. Food Chem. 2008, 56, 2199–2207. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B. Solution and conformational properties of gum kondagogu (Cochlospermum gossypium)–A natural product with immense potential as a food additive. Food Chem. 2009, 116, 686–692. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sreedhar, B.; Rama Rao, B.; Nageswara Rao, T.; Johny, T.A. Interaction of Pb2+ and Cd2+ with gum kondagogu (Cochlospermum gossypium): A natural carbohydrate polymer with biosorbent properties. Carbohyd. Polym. 2009, 78, 894–901. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sukumar, A.A. Competitive adsorption of toxic heavy metal contaminants by gum kondagogu (Cochlospermum gossypium): A natural hydrocolloid. Colloids Surf. B 2010, 75, 490–495. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sreedhar, B. Biosorption of nickel and total chromium from aqueous solution by gum kondagogu (Cochlospermum gossypium): A carbohydrate biopolymer. J. Hazard. Mat. 2010, 178, 851–860. [Google Scholar] [CrossRef]
- Naidu, V.G.M.; Madhusudhana, K.; Sashidhar, R.B.; Ramakrishna, S.; Khar, V.; Ahmed, F.J.; Diwan, P.V. Polyelectrolyte complexes of gum kondagogu and chitosan, as diclofenac carriers. Carbohyd. Polym. 2009, 76, 464–471. [Google Scholar] [CrossRef]
- Naidu, V.G.M.; Ramakrishna, S.; Palaniappan, S.; Sashidhar, R.B.; Khar, R.K.; Diwan, P.V. Emulsifying properties of gum kondagogu (Cochlospermum gossypium), a natural biopolymer. J. Sci. Food Agric. 2010, 89, 1271–1276. [Google Scholar]
- Rivas, B.L.; Seguel, G.V.; Ancatripai, C. Polymer–metal complexes: Synthesis, characterization, and properties of poly (maleic acid) metal complexes with Cu(II), Co(II), Ni(II) and Zn(II). Polym. Bull. 2000, 44, 445–452. [Google Scholar] [CrossRef]
- Trimukhe, K.D.; Varma, A.J. Complexation of heavy metals by cross-linked chitin and its deacetylated derivatives. Carbohyd. Polym. 2008, 71, 66–73. [Google Scholar] [CrossRef]
- Lazaro, N.; Sevilla, A.L.; Morales, S.; Marques, A.M. Heavy metal biosorption by gellan gum gel beads. Water Res. 2003, 37, 2118–2126. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B.; Sivaprasad, N.; Sarma, U.V.M.; Satyanarayana, N.; Kumaresan, R.; Nagaeswara Rao, T.; Raviprasad, P. Bioremediation of mercury (II) from aqueous solution by gum karaya (Sterculia urens): A natural hydrocolloid. Desalination 2011, 272, 270–277. [Google Scholar] [CrossRef]
- Gok, C.; Aytas, S. Biosorption of uranium (VI) from aqueous solution using calcium alginate beads. J. Hazard. Mater. 2009, 168, 369–375. [Google Scholar] [CrossRef]
- Utgikar, V.; Chen, B.Y.; Tabak, H.H.; Bishop, D.F.; Govind, R. Treatment of acid mine drainage, I: Equilibrium biosorption of zinc and copper on non-viable activated sludge. Int. Biodeterior. Biodegrad. 2000, 46, 19–28. [Google Scholar] [CrossRef]
- Ikeda, S.; Funami, T.; Zhang, G. Visualizing surface-active hydrocolloids by atomic force microscopy. Carbohydr. Polym. 2005, 62, 192–196. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Guo, S. Mechanisms of lead biosorption on cellulose/chitin beads. Water Res. 2005, 39, 3755–3762. [Google Scholar] [CrossRef]
- Glatter, O. Determination of particle-size distribution functions from small-angle scattering data by means of the indirect transformation method. J. Appl. Crystl. 1980, 13, 7–11. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, G. Small Angle X-ray Scattering; Academic Press Inc: London, UK, 1982. [Google Scholar]
- Glatter, O.; Hainisch, B. Improvements in real-space deconvolution of small-angle scattering data. J. Appl. Crystallogr. 1984, 17, 435–441. [Google Scholar] [CrossRef]
- Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Cryst. 1972, 10, 415–421. [Google Scholar] [CrossRef]
- Tao, F.; Biao, G.Z.; Yu, J.Z.; Ning, Z.H. Isolation and charactrization of an acidic polysaccharide from Mesona Blumes gum. Carbohydr. Polym. 2008, 71, 159–169. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the kondagogu gum and its metal complexes are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vinod, V.T.P.; Sashidhar, R.B.; Černík, M. Morphology and Metal Binding Characteristics of a Natural Polymer—Kondagogu (Cochlospermum gossypium) Gum. Molecules 2013, 18, 8264-8274. https://doi.org/10.3390/molecules18078264
Vinod VTP, Sashidhar RB, Černík M. Morphology and Metal Binding Characteristics of a Natural Polymer—Kondagogu (Cochlospermum gossypium) Gum. Molecules. 2013; 18(7):8264-8274. https://doi.org/10.3390/molecules18078264
Chicago/Turabian StyleVinod, V. T. P., R. B. Sashidhar, and Miroslav Černík. 2013. "Morphology and Metal Binding Characteristics of a Natural Polymer—Kondagogu (Cochlospermum gossypium) Gum" Molecules 18, no. 7: 8264-8274. https://doi.org/10.3390/molecules18078264