Enhanced Stability of CaO and/or La2O3 Promoted Pd/Al2O3 Egg-Shell Catalysts in Partial Oxidation of Methane to Syngas
Abstract
:1. Introduction
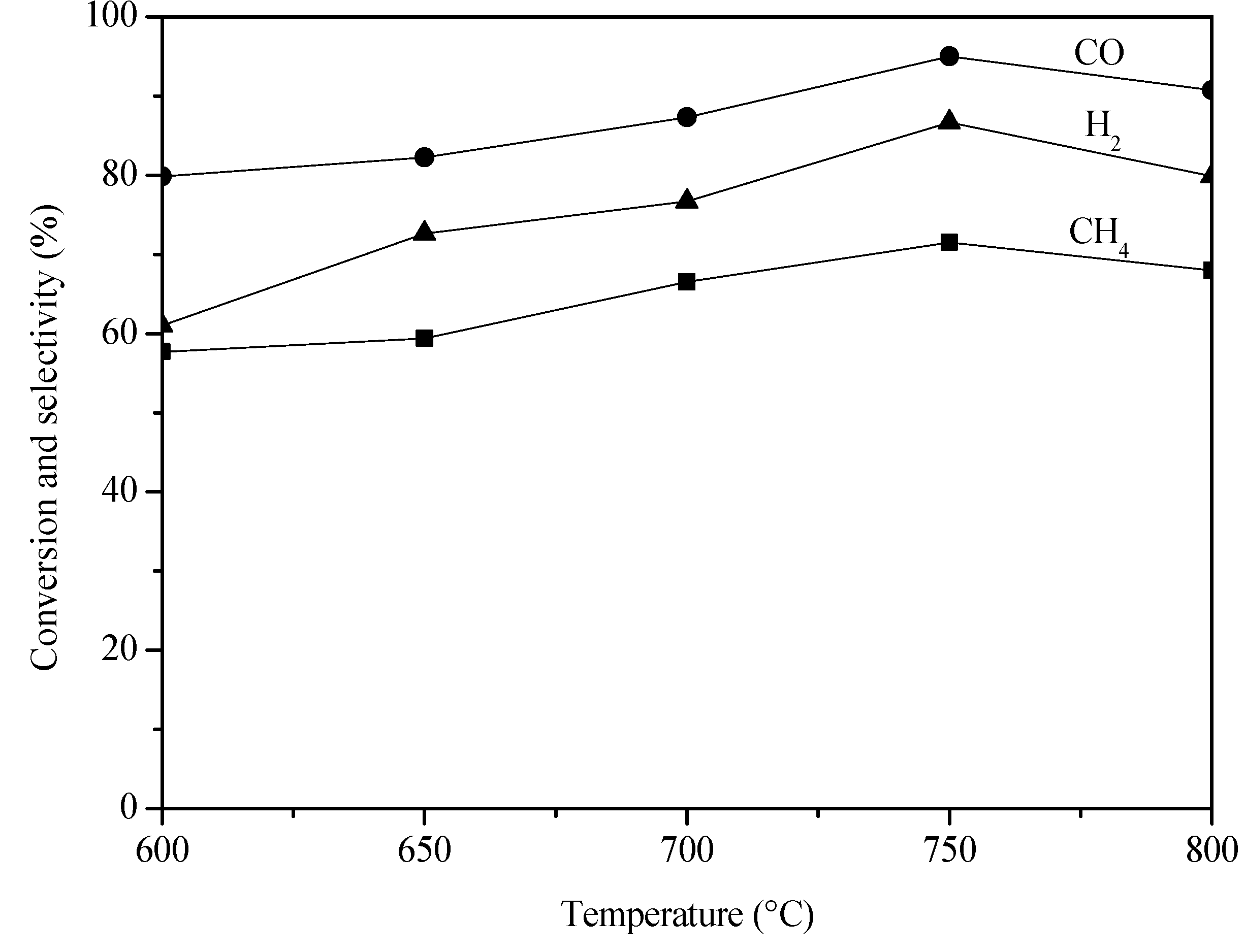
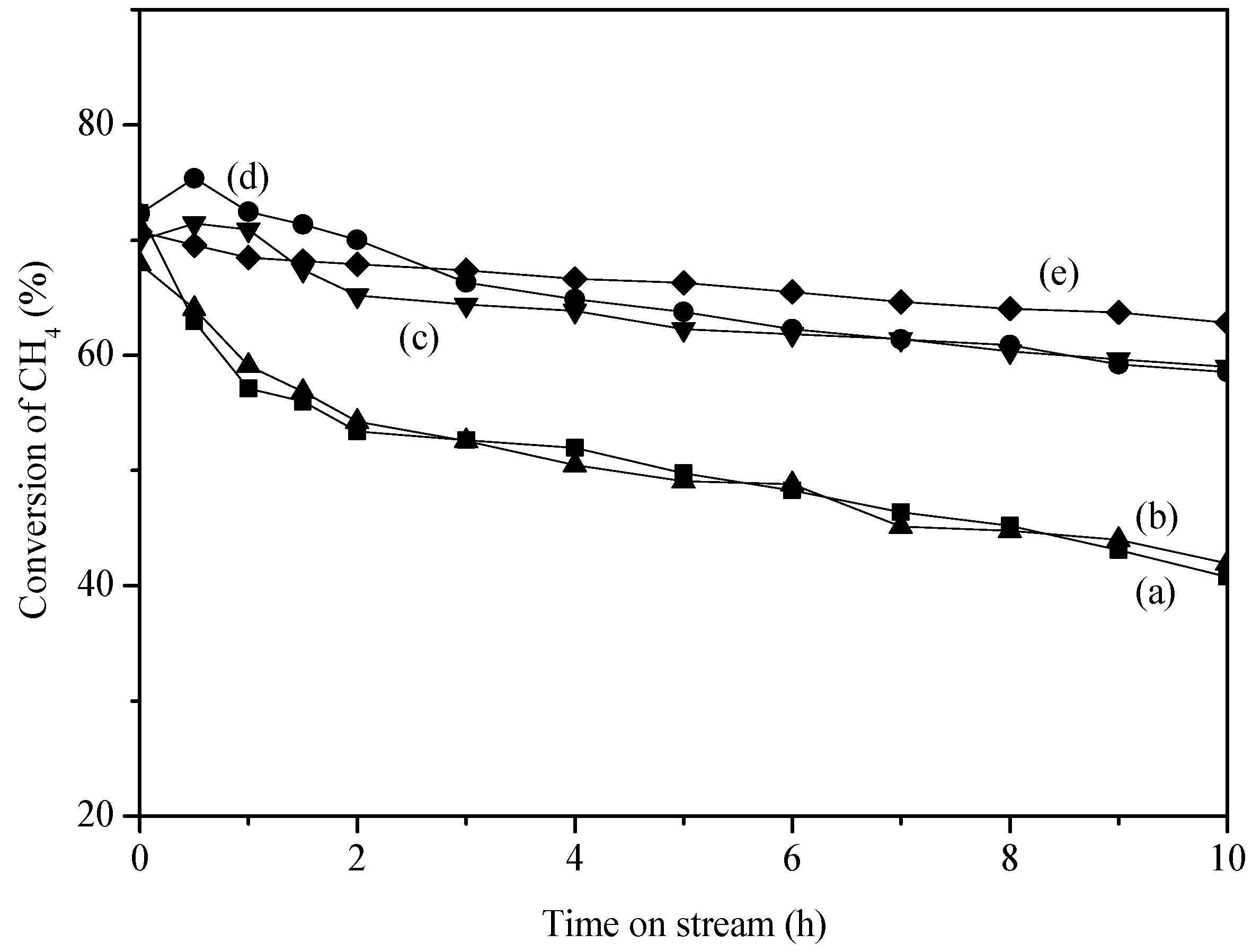
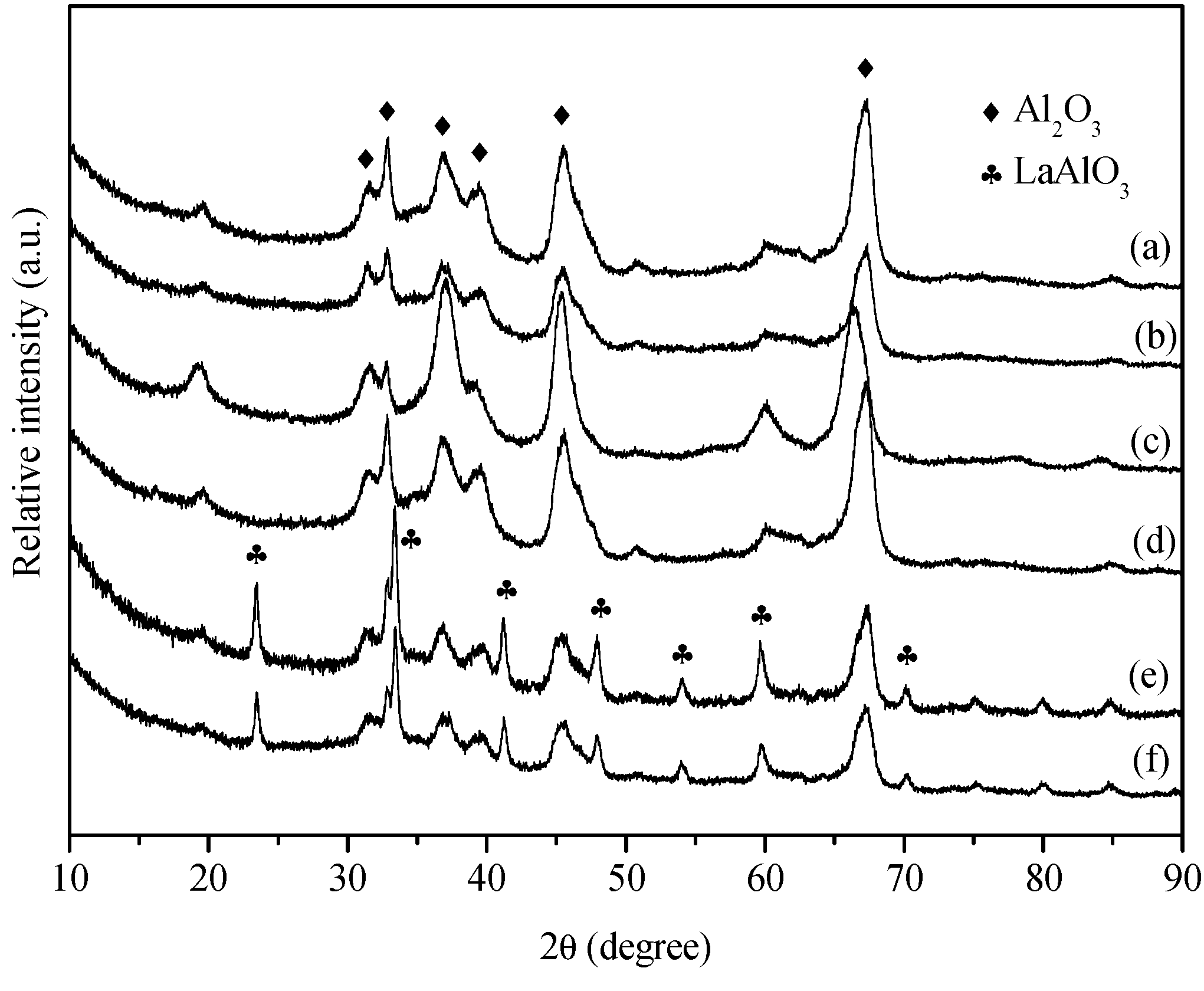
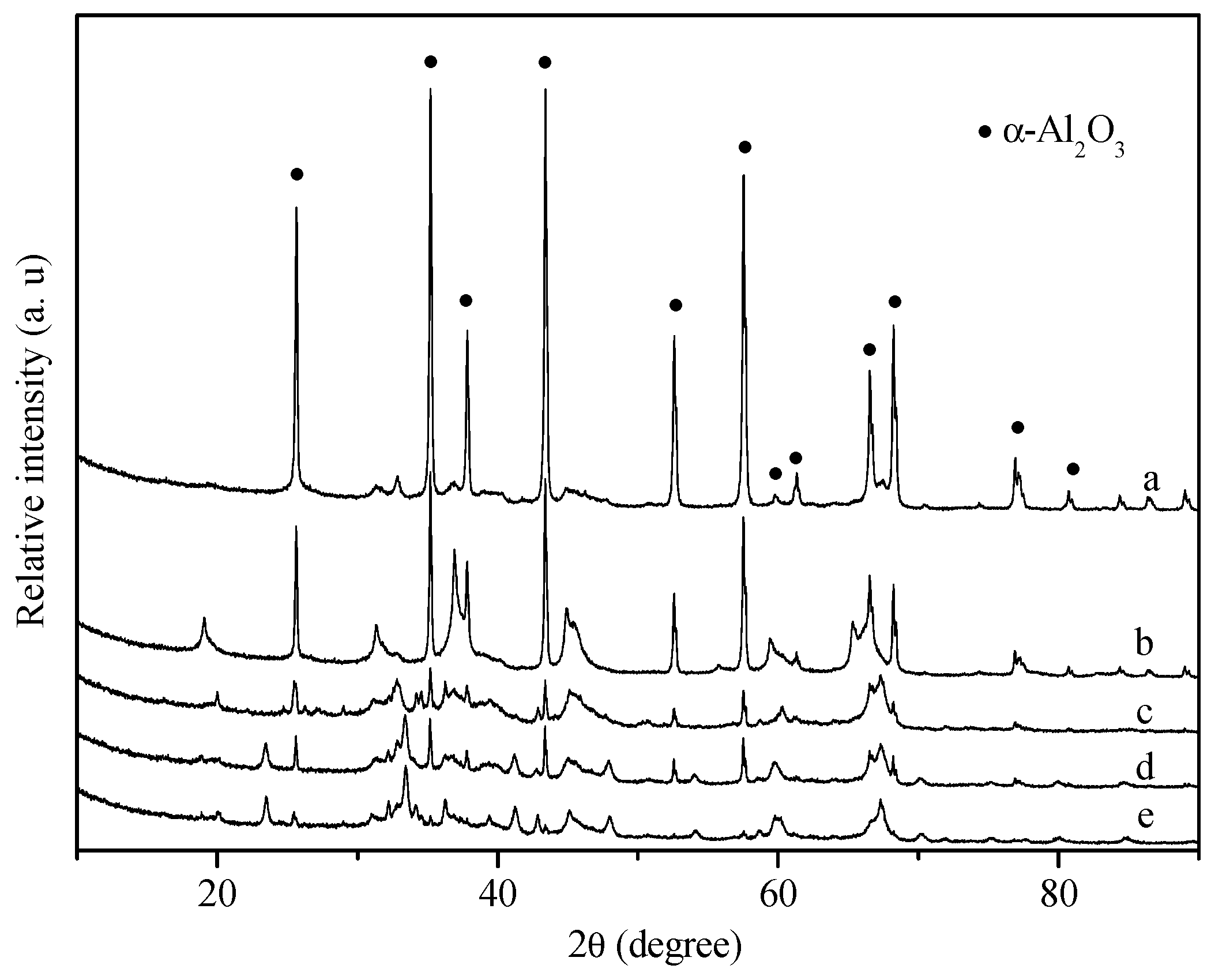
2. Results and Discussion
| Sample | Specific surface area (m2/g) |
|---|---|
| Al2O3 | 118 |
| Pd/Al2O3 | 58 |
| Pd/MgO/Al2O3 | 99 |
| Pd/CaO/Al2O3 | 78 |
| Pd/La2O3/Al2O3 | 78 |
| Pd/La2O3/CaO/Al2O3 | 102 |


3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bitsch-Larsen, A.; Horn, R.; Schmidt, L.D. Catalytic partial oxidation of methane on rhodium and platinum: Spatial profiles at elevated pressure. Appl. Catal. A 2008, 348, 165–172. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Li, B.T.; Kunimori, K.; Suzuki, K.; Fujimoto, K.; Tomishige, K. Additive effect of noble metals on NiO-MgO solid solution in oxidative steam reforming of methane under atmospheric and pressurized conditions. Appl. Catal. A 2006, 299, 145–156. [Google Scholar] [CrossRef]
- Kikuchi, E.; Chen, Y. Syngas formation by partial oxidation of methane in palladium membrane reactor. Stud. Surf. Sci. Catal. 1998, 119, 441–446. [Google Scholar] [CrossRef]
- Qin, D.Y.; Lapszewicz, J.; Jiang, X.Z. Comparison of partial oxidation and steam-CO2 mixed reforming of CH4 to syngas on MgO-supported metals. J. Catal. 1996, 159, 140–149. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Tsang, S.C.; Claridge, J.B.; Green, M.L.H. Recent advances in the conversion of methane to synthesis gas. Catal. Today 1995, 23, 3–15. [Google Scholar] [CrossRef]
- Lanza, R.; Velascoa, J.A.; Järås, S.G. Recent Developments and Achievements inPartial Oxidation of Methane with andwithout Addition of Steam. In Catalysis; Spivey, J.J., Dooley, K.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2011; Volumn 23, pp. 50–95. [Google Scholar]
- Yang, S.W.; Kondo, J.N.; Hayashi, K.; Hirano, M.; Domen, K.; Hosono, H. Partial oxidation of methane to syngas over promoted C12A7. Appl. Catal. A 2004, 277, 239–246. [Google Scholar] [CrossRef]
- Choque, V.; Homs, N.; Cicha-Szot, R.; de la Piscina, P.R. Study of ruthenium supported on Ta2O5-ZrO2 and Nb2O5-ZrO2 as catalysts for the partial oxidation of methane. Catal. Today 2009, 142, 308–313. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Prabhakar, B.; Rajput, A.M.; Mamman, A.S. Oxidative conversion of methane to CO and H2 over Pt or Pd containing alkaline and rare earth oxide catalysts. Fuel 1998, 77, 1477–1481. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Breach, J.A.; Chand, S.; Ghorai, D.K.; Hartridge, A.; Keary, J.; Mallick, K.K. Selective oxidation of methane to carbon monoxide on supported palladium catalyst. Appl. Catal. A 1992, 80, L1–L5. [Google Scholar]
- Ryu, J.H.; Lee, K.Y.; Kim, H.J.; Yang, J.I.; Jung, H. Promotion of palladium-based catalysts on metal monolith for partial oxidation of methane to syngas. Appl. Catal. B 2008, 80, 306–312. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Chen, J.X.; Zhang, J.Y. Effect of calcination temperature on properties of eggshell Ni/MgO-Al2O3 catalyst for partial oxidation of methane to syngas. Catal. Lett. 2009, 127, 312–328. [Google Scholar] [CrossRef]
- Vella, L.D.; Montini, T.; Specchia, S.; Fornasiero, P. Fixed beds of Rh/Al2O3-based catalysts for syngas production in methane SCT-GPO reactors. Int. J. Hydrogen Energy 2011, 36, 7776–7784. [Google Scholar] [CrossRef]
- Specchia, S.; Vella, L.D.; de Rogatis, L.; Montini, T.; Specchia, V.; Fornasiero, P. Rh-based catalysts for syngas production via SCT-CPO reactors. Catal. Today 2010, 155, 101–107. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Chen, J.X.; Zhang, J.Y. Influence of nickel distribution on properties of spherical catalysts for partial oxidation of methane to synthesis gas. Catal. Commun. 2007, 8, 508–512. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Chen, J.X.; Zhang, J.Y. Effects of CeO2 and CaO composite promoters on the properties of eggshell Ni/MgO-Al2O3 catalysts for partial oxidation of methane to syngas. React. Kinet. Catal. Lett. 2008, 94, 351–357. [Google Scholar] [CrossRef]
- Liu, Z.N.; Zhou, S.H.; Hou, M.B.; Xie, Z.K.; Zhu, H.Y. First-stage selective hydrogenation catalyst for pyrolysis gasaline. Chinese Patent CN1443829A, 24 September 2003. [Google Scholar]
- Dissanayake, D.; Rosynek, M.P.; Kharas, K.C.C.; Lunsford, J.H. Partial oxidation of methane to carbon monoxide and hydrogen over a Ni/Al2O3 catalyst. J. Catal. 1991, 132, 117–127. [Google Scholar]
- Zhang, Z.L.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over supported Ni catalysts. Catal. Today 1994, 21, 589–595. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xiong, G.X.; Sheng, S.S.; Yang, W.S. Deactivation studies over NiO/γ-Al2O3 catalysts for partial oxidation of methane to syngas. Catal. Today 2000, 63, 517–522. [Google Scholar] [CrossRef]
- Jing, S.Y.; Lin, L.B.; Huang, N.K.; Zhang, J.; Lu, Y. Investigation on lattice constants of Mg-Al spinels. J. Mater. Sci Lett. 2000, 19, 225–227. [Google Scholar] [CrossRef]
- Shi, C.K.; Zhang, P. Effect of a second metal (Y, K, Ca, Mn or Cu) addition on the carbon dioxide reforming of methane over nanostructured palladium catalysts. Appl. Catal. B 2012, 115–116, 190–200. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Yu, H.; Ma, Z.; Zhou, S. Enhanced Stability of CaO and/or La2O3 Promoted Pd/Al2O3 Egg-Shell Catalysts in Partial Oxidation of Methane to Syngas. Molecules 2013, 18, 8289-8297. https://doi.org/10.3390/molecules18078289
Wang J, Yu H, Ma Z, Zhou S. Enhanced Stability of CaO and/or La2O3 Promoted Pd/Al2O3 Egg-Shell Catalysts in Partial Oxidation of Methane to Syngas. Molecules. 2013; 18(7):8289-8297. https://doi.org/10.3390/molecules18078289
Chicago/Turabian StyleWang, Jinlong, Hongbo Yu, Zhen Ma, and Shenghu Zhou. 2013. "Enhanced Stability of CaO and/or La2O3 Promoted Pd/Al2O3 Egg-Shell Catalysts in Partial Oxidation of Methane to Syngas" Molecules 18, no. 7: 8289-8297. https://doi.org/10.3390/molecules18078289






