Dendrimers in Layer-by-Layer Assemblies: Synthesis and Applications
Abstract
:1. Introduction
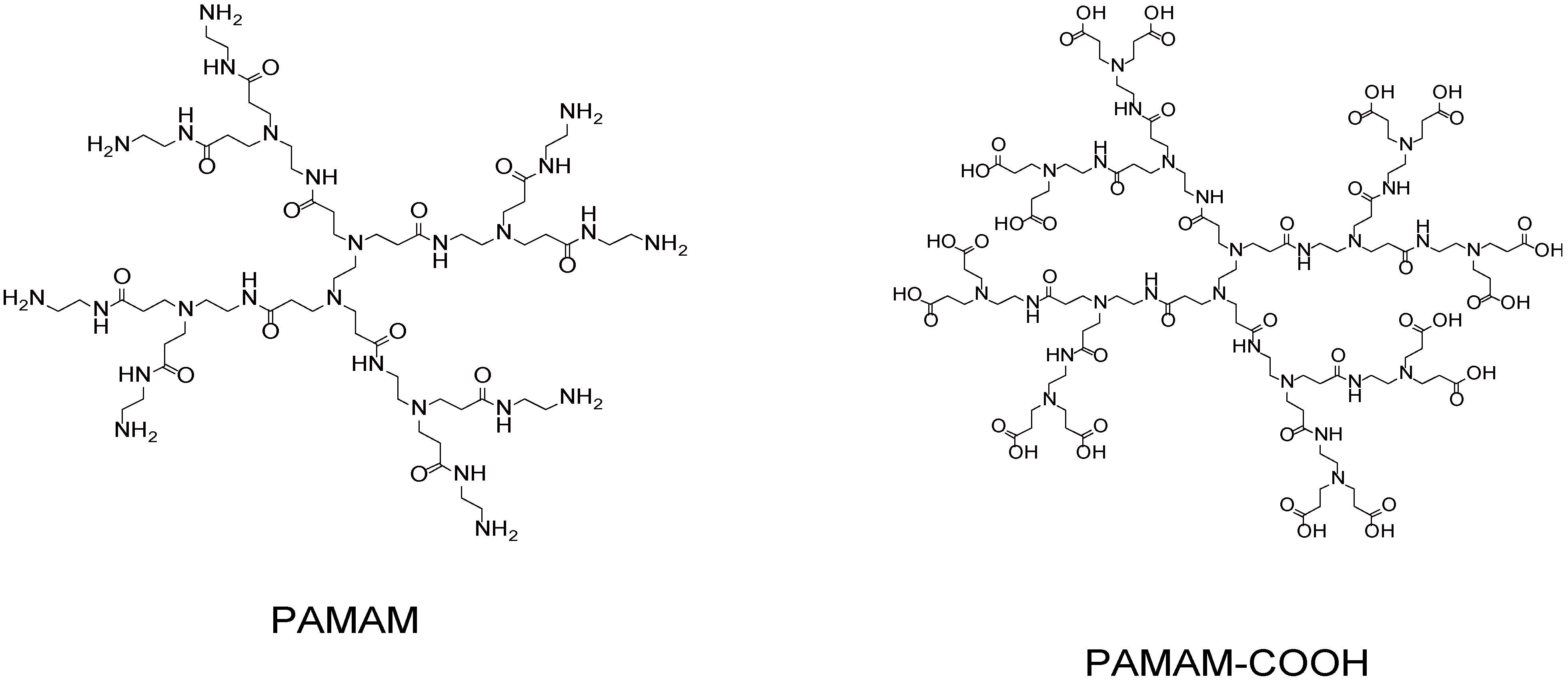
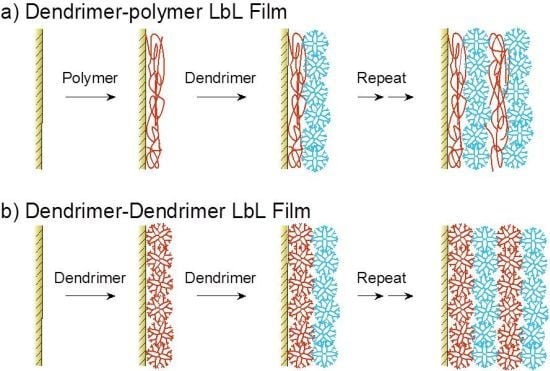
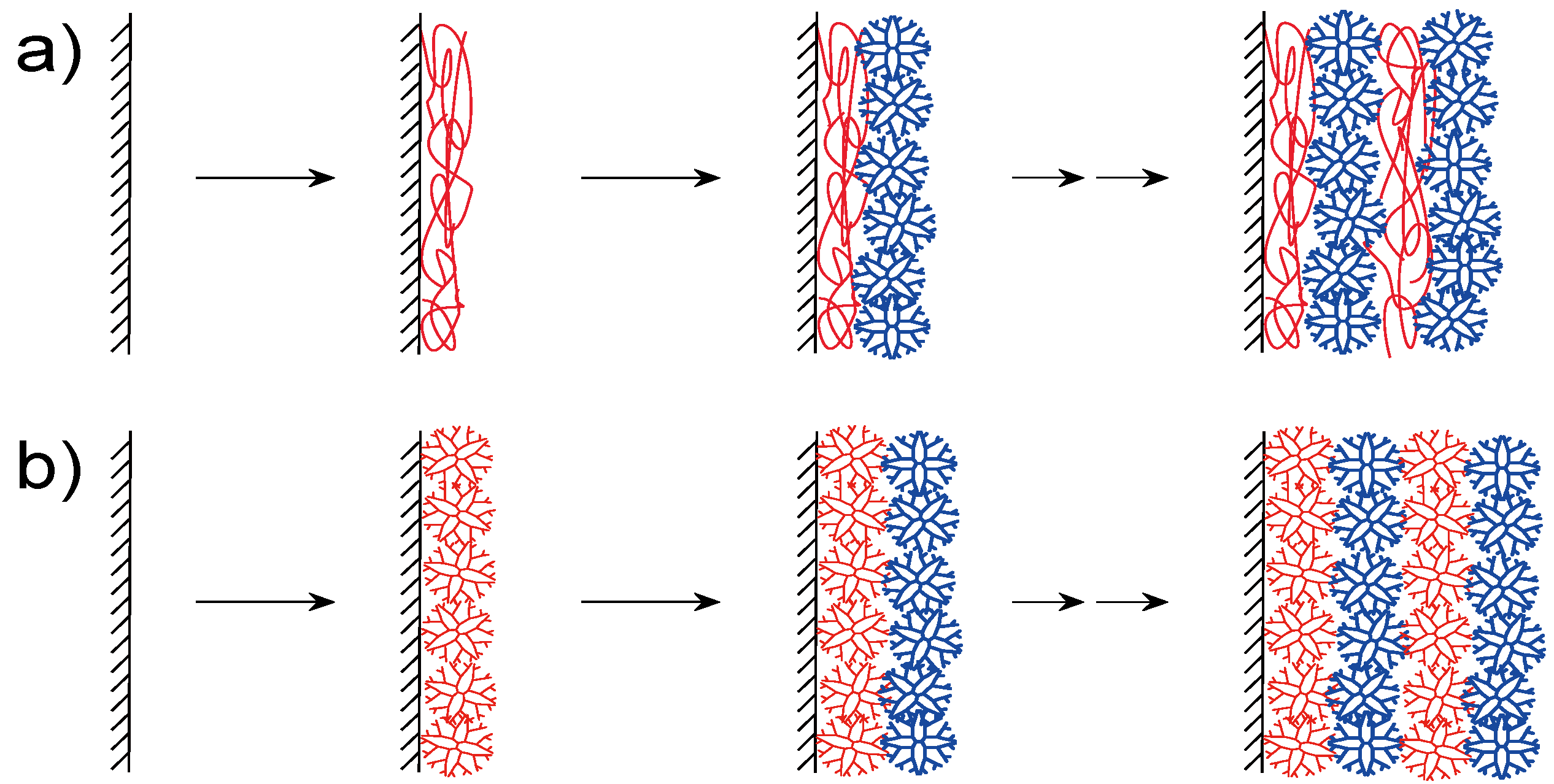
2. Synthesis of dendrimer LbL assemblies
2.1. Electrostatic Bonding LbL Films
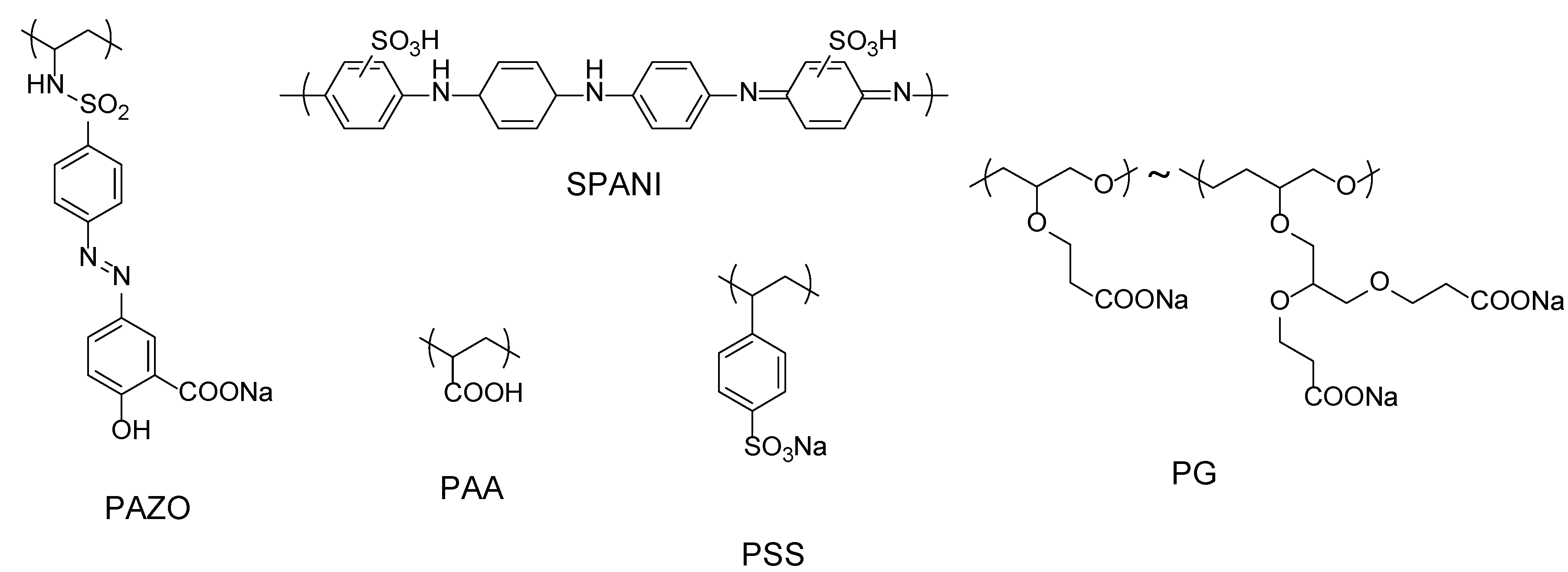
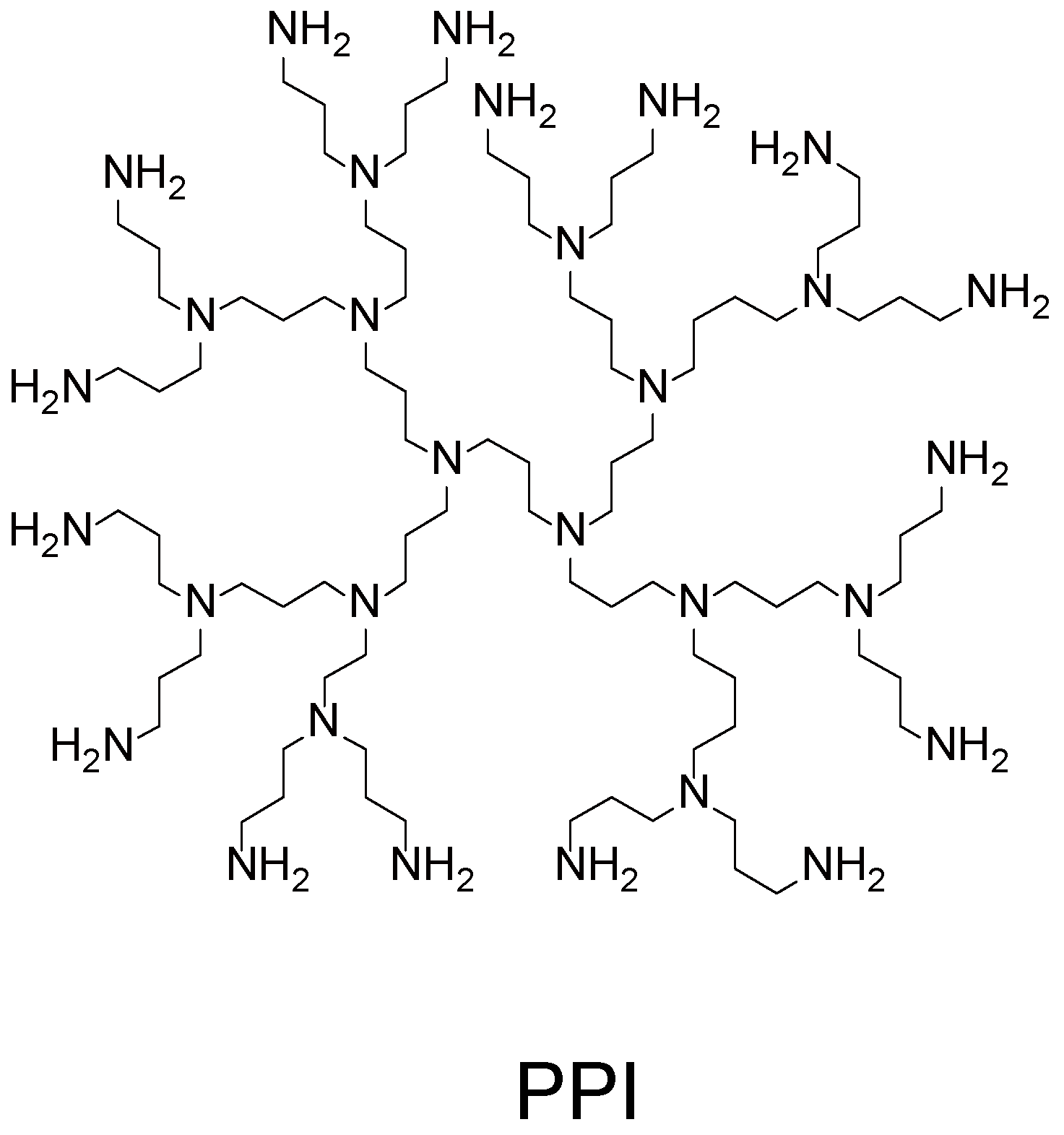
2.2. Hydrogen-Bonding LbL Films
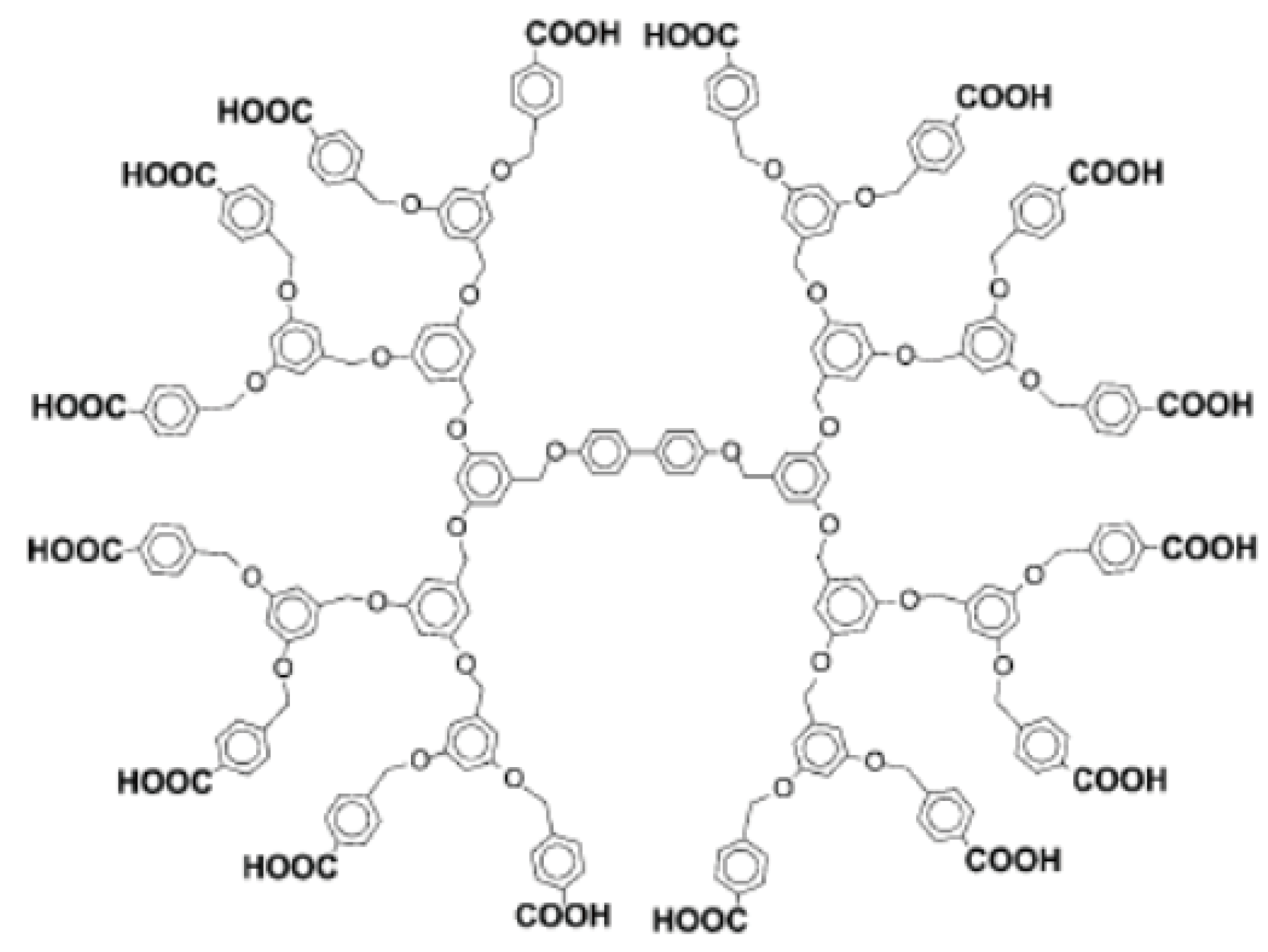
2.3. Covalent-Bonding LbL Films
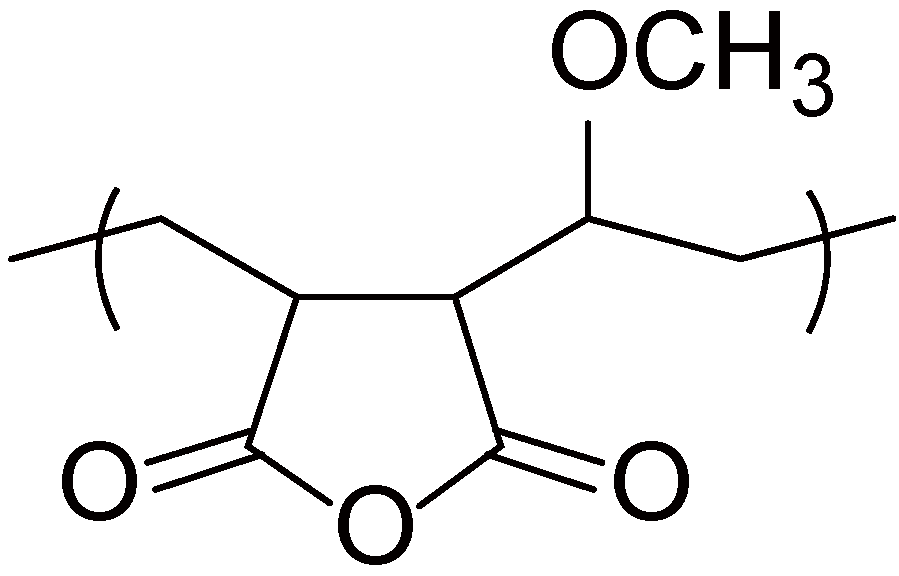
2.4. Miscellaneous
3. Applications of Dendrimer LbL Assemblies
3.1. Biosensors
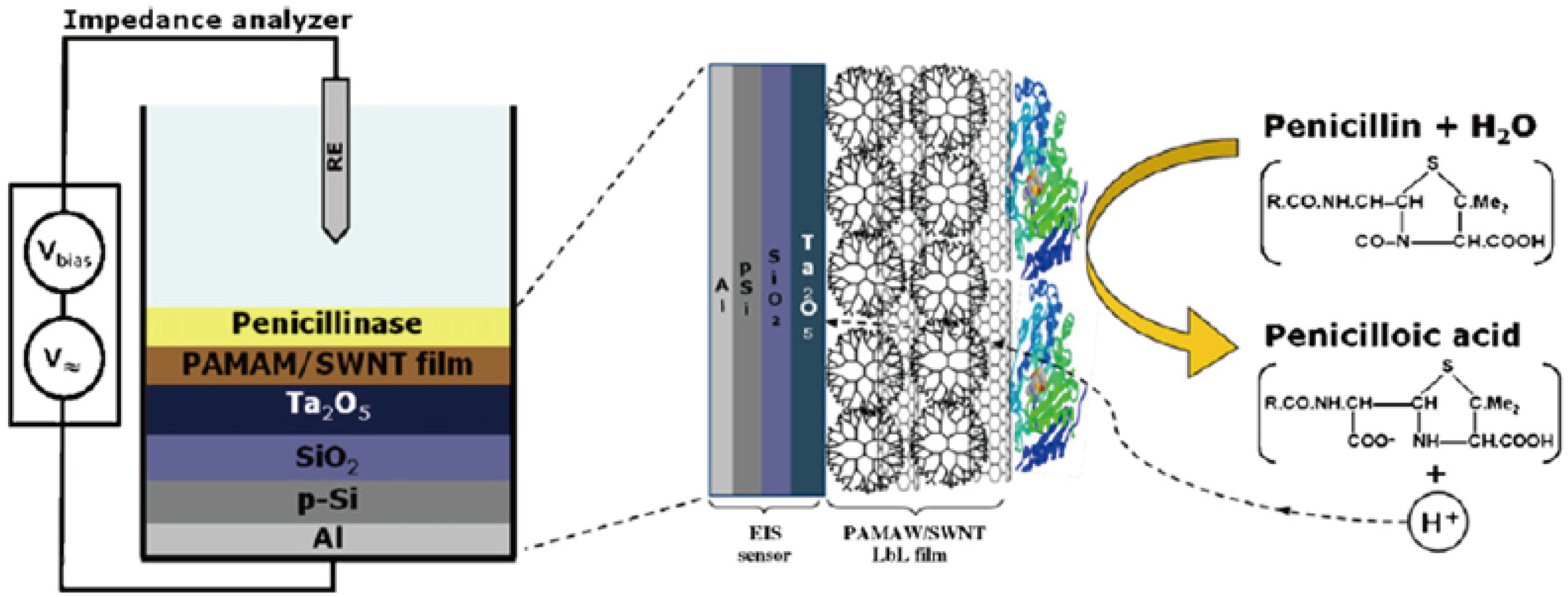
3.2. Encapsulation and Controlled Release
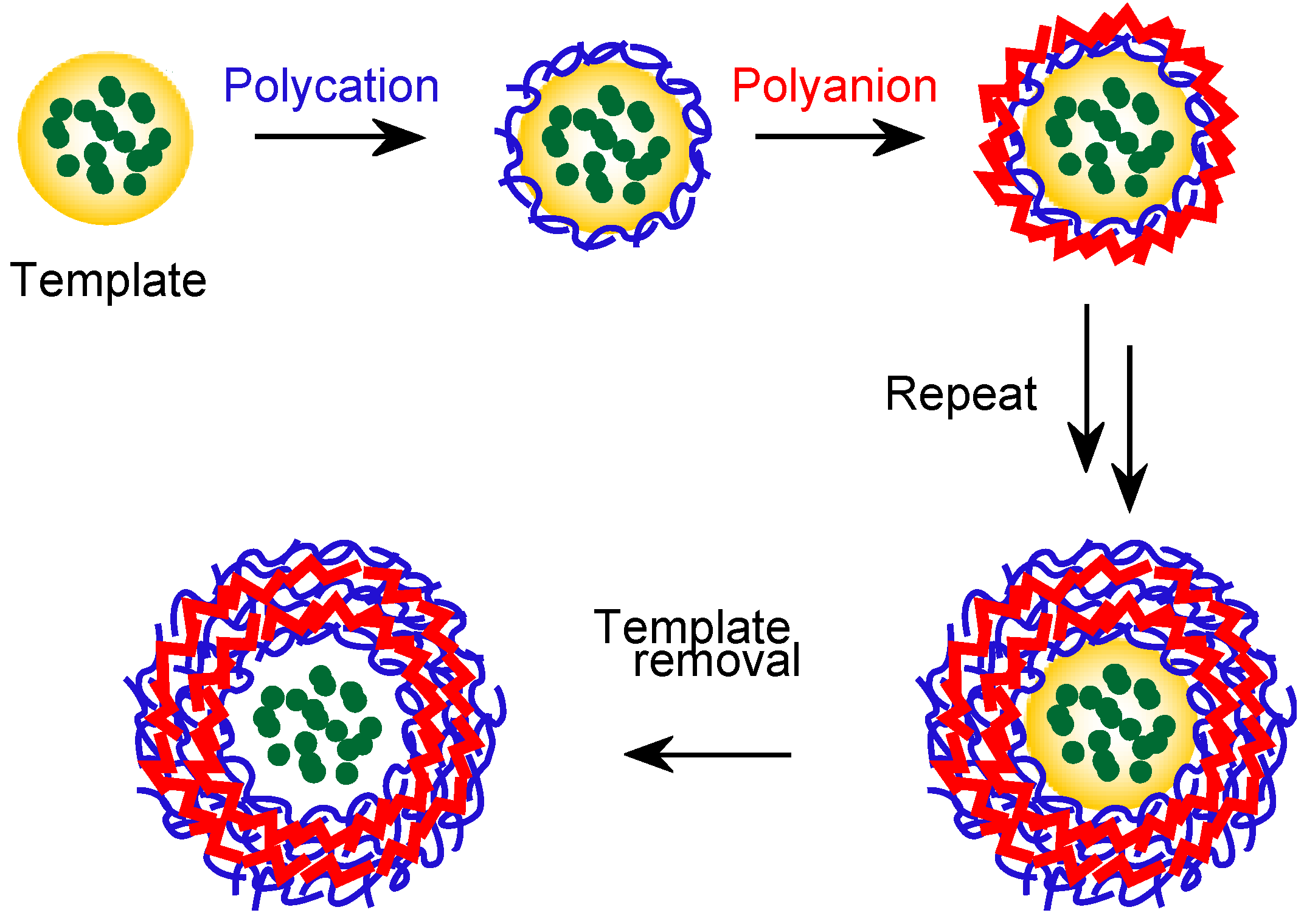
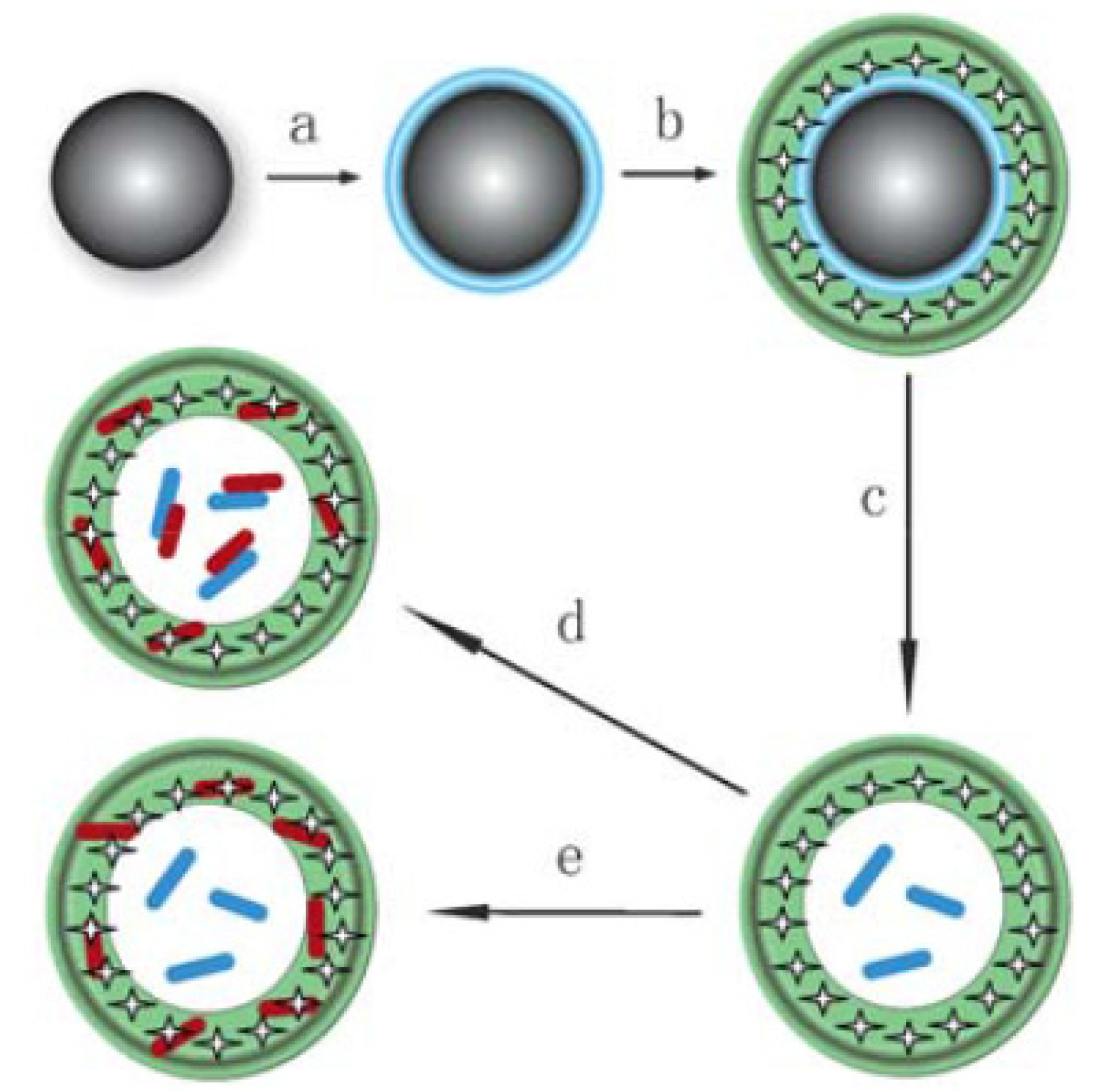
3.3. Miscellaneous
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst–dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Newcome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Cascade molecules: A new approach to micelles, a [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A., III. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Eng. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Progr. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; de Brabander-van den Berg, E.M.M.; Meijer, E.W. Encapsulation of guest molecules into a dendritic box. Science 1994, 266, 1226–1229. [Google Scholar]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef]
- Aoi, K.; Itoh, K.; Okada, M. Globular carbohydrate macromolecule “sugar balls”. 1. Synthesis of novel sugar-persubstituted poly(amido amine) dendrimers. Macromolecules 1995, 28, 5391–5393. [Google Scholar] [CrossRef]
- Zhu, S.; Hong, M.; Zhang, L.; Tang, G.; Jiang, Y.; Pei, Y. PEGylated PAMAM dendrimer-doxorubicin conjugates: In vitro evaluation and in vivo tumor accumulation. Pharm. Res. 2009, 27, 161–174. [Google Scholar]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Iman, M.R.; Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Ramireddy, R.R.; Raghupathi, K.R.; Torres, D.A.; Thayumanavan, S. Stimuli sensitive amphiphilic dendrimers. New J. Chem. 2012, 36, 340–349. [Google Scholar] [CrossRef]
- Tian, W.; Ma, Y. Theoretical and computational studies of dendrimers as delivery vectors. Chem. Soc. Rev. 2013, 42, 705–727. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Beezer, A.E.; King, A.S.H.; Martin, I.K.; Mitchel, J.C.; Twyman, L.J.; Wain, C.F. Dendrimers as potential drug carriers; encapsulation of acidic hydrophobes within water soluble PAMAM dendrimers. Tetrahedron 2003, 59, 3873–3880. [Google Scholar] [CrossRef]
- Morgan, E.J.; Rippy, J.M.; Tucer, S.A. Spectroscopic characterization of poly(amidoamine) dendrimers as selective uptake devices: Phenol blue versus Nile red. Appl. Spectr. 2006, 60, 551–559. [Google Scholar] [CrossRef]
- Gurdag, S.; Khandare, J.; Stapels, S.; Matherly, L.H.; Kannan, R.M. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and resistant cell lines. Bioconjug. Chem. 2006, 17, 275–283. [Google Scholar] [CrossRef]
- Majoros, I.J.; Myc, A.; Thomas, T.; Mehta, C.B.; Baker, J.R., Jr. PAMAM dendrimer–based multifunctional conjugate for cancer therapy: Synthesis, characterization, and functionality. Biomacromolecules 2006, 7, 572–579. [Google Scholar] [CrossRef]
- Kazzouli, S.E.; Mignani, S.; Bousmina, M.; Majoral, J.-P. Dendrimer therapeutics: Covalent and ionic attachments. New J. Chem. 2012, 36, 227–240. [Google Scholar] [CrossRef]
- Ainalem, M.-L.; Nylander, T. DNA condensation using cationic dendrimers-morphology and molecular structure of formed aggregates. Soft Matt. 2011, 7, 4577–4594. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Betcheva, R. Fluorescent dendrimers as sensors for biologically important metal cations. Curr. Med.l Chem. 2012, 19, 4976–4983. [Google Scholar] [CrossRef]
- Tang, J.; Sheng, Y.; Hu, H.; Shen, Y. Macromolecular MRI contrast agents: Structures, properties and applications. Progr. Polym. Sci. 2013, 38, 462–502. [Google Scholar] [CrossRef]
- Satija, J.; Sai, V.V.R.; Mukherji, S. Dendrimers in biosensors: Concept and applications. J. Mater. Chem. 2011, 21, 14367–14386. [Google Scholar] [CrossRef]
- Karadag, M.; Geyik, C.; Demerkol, D.O.; Ertas, F.N.; Timur, S. Modified gold surface by 6-(ferrocenyl)hexanethiol/dendrimer/gold nanoparticles as a platform for the mediated biosensing applications. Mater. Sci. Eng. C 2013, 33, 634–640. [Google Scholar] [CrossRef]
- Ujihira, M.; Imae, T. Hierarchical structures of dendritic polymers. Polym. Int. 2010, 59, 37–144. [Google Scholar]
- Kano, K. Dendrimer-based bionanomaterials produced by surface modification, assembly and hybrid formation. Polym. J. 2012, 44, 531–540. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buuildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Lvov, Y.; Decher, G.; Möhwald, H. Assembly, structural characterization, and thermal behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulfate) and poly(allylamine). Langmuir 1993, 9, 481–486. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH–Dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer films: Effects of electrochemically inactive surface layers on the redox properties. Langmuir 2003, 19, 4043–4046. [Google Scholar] [CrossRef]
- Sato, K.; Suzuki, I.; Anzai, J. Preparation of polyelectrolyte-layered assemblies containing cyclodextrin and their binding properties. Langmuir 2003, 19, 7406–7412. [Google Scholar] [CrossRef]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.-C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Sato, H.; Anzai, J. Preparation of layer-by-layer thin films composed of DNA and ferrocene-bearing poly(amine)s and their redox properties. Biomacromolecules 2006, 7, 2072–20767. [Google Scholar] [CrossRef]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 21, 8354–8359. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, K.; Anzai, J. Layer–by–layer polyelectrolyte films containing insulin for pH-triggered release. J. Mater. Chem. 2010, 20, 1546–1552. [Google Scholar]
- Vogt, C.; Mertz, D.; Benmlih, K.; Hemmerlé, J.; Voegel, J.-C.; Schaaf, P.; Lavalle, P. Layer-by-layer enzymatic platform for stretched-induced reactive release. ACS Macro Lett. 2012, 1, 797–801. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Ito, S.; Tsutsumi, N. Adsorption–induced second harmonic generation from the layer–by–layer deposited ultrathin film based on the charge–transfer interaction. Langmuir 2000, 16, 9478–9482. [Google Scholar] [CrossRef]
- Ogawa, Y.; Arikawa, Y.; Kida, T.; Akashi, M. Fabrication of layer-by-layer assembly films composed of poly(lactic acid) and polylysine through cation–dipole interactions. Langmuir 2008, 24, 8606–8609. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Granick, S. Layered erasable polymer multilayers formed by hydrogen-bonded sequential self–assembly. Macromolecules 2002, 35, 301–310. [Google Scholar] [CrossRef]
- Buck, M.E.; Lynn, D.M. Free-standing and reactive thin films fabricated by covalent layer-by-layer assembly and subsequent lift–off of azlactone-containing polymer multilayers. Langmuir 2010, 26, 16134–16140. [Google Scholar] [CrossRef]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively-charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun. 2002, 164–165. [Google Scholar]
- Rydzek, G.; Parat, A.; Polavarapu, P.; Baehr, C.; Voegel, J.-C.; Hemmerlé, J.; Senger, B.; Frisch, B.; Schaaf, P.; Jierry, L.; et al. One–pot morphogen driven self–constructing films based on non–covalent host–guest interactions. Soft Matt. 2012, 8, 446–453. [Google Scholar] [CrossRef]
- Bourdillon, C.; Demaille, C.; Moiroux, J.; Savéant, J.-M. From homogeneous electroenzymatic kinetics to antigen–antibody construction and characterization of spatially ordered catalytic enzyme assemblies on electrodes. Acc. Chem. Res. 1996, 29, 529–535. [Google Scholar] [CrossRef]
- Hoshi, T.; Akase, S.; Anzai, J. Preparation of multilayer thin films containing avidin through sugar–lectin interactions and their binding properties. Langmuir 2002, 18, 7024–7028. [Google Scholar] [CrossRef]
- Yao, H.; Hu, N. pH–controllable on–off bioelectroctalysis of bienzyme; layer-by-layer films assembled by cancanavalin A and glycoenzymes with an electroactive mediator. J. Phys. Chem. B 2010, 114, 9926–9933. [Google Scholar]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer–by–layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Sukhishvili, S.A. Layer-by-layer hydrogen-bonded polymer films: From fundamentals to applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Imae, T. Fabrication of dendrimer toward biological applications. Progr. Mol. Biol. Trans. Sci. 2011, 104, 101–139. [Google Scholar] [CrossRef]
- Hammond, P.T. Engineering materials layer-by-layer: Challenges and opportunities in multilayer assembly. AIChE J. 2011, 57, 2928–2940. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte multilayer assemblies on materials surface: From cell adhesion to tissue engineering. Chem. Mater. 2011, 24, 854–869. [Google Scholar]
- Takahashi, S.; Sato, K.; Anzai, J. Layer–by–layer construction of protein architectures through avidin–biotin and lectin–sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef]
- Hammond, P.T. Building biomedical materials layer-by-layer. Mater. Today 2012, 15, 196–206. [Google Scholar] [CrossRef]
- De Koker, S.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, L.; Crooks, R.M. Determination of the intrinsic proton binding constants for poly(amidoamine) dendrimers via potentiometric pH titration. Macromolecules 2003, 36, 5725–5731. [Google Scholar] [CrossRef]
- Tsukruk, V.; Rinderspacher, F.; Bliznyuk, V.N. Self-assembled multilayer films from dendrimers. Langmuir 1997, 13, 2171–2177. [Google Scholar] [CrossRef]
- Casson, J.L.; Wang, H.-L.; Roberts, J.B.; Parikh, A.N.; Robinson, J.M.; Johal, M.S. Kinetics and interpenetration of ionically self-assembled dendrimer and PAZO. J. Phys. Chem. B 2002, 106, 1697–1702. [Google Scholar] [CrossRef]
- Kim, B.Y.; Bruening, M.L. pH-dependent growth and morphology of multilayer dendrimer/poly(acrylic acid) films. Langmuir 2003, 19, 94–99. [Google Scholar] [CrossRef]
- Li, C.; Mitamura, K.; Imae, T. Electrostatic layer-by-layer assembly of poly(amido amine) dendrimer/conducting sulfonated polyaniline: Structure and properties of multilayer films. Macromolecules 2003, 36, 9957–9965. [Google Scholar] [CrossRef]
- Khopade, A.J.; Caruso, F. Investigation of the factors influencing the formation of dendrimer/polyanion multilayer films. Langmuir 2002, 18, 7669–7676. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, O.-J.; Barriau, E.; Li, X.; Caminade, A.-M.; Majoral, J.-P.; Frey, H.; Knoll, W. Hybrid organic-inorganic nanostructures fabricated from layer-by-layer self–assembled multilayers of hyperbranched polyglycerols and phosphorus dendrimers. J. Nanosci. Nanotechnol. 2006, 6, 3871–3876. [Google Scholar] [CrossRef]
- He, J.-A.; Valluzzi, R.; Yang, K.; Dolukhanyan, T.; Sung, C.; Kumar, J.; Tripathy, S.K. Electrostatic multilayer deposition of a gold-dendrimer nanocomposite. Chem. Mater. 1999, 11, 3268–3274. [Google Scholar] [CrossRef]
- Esumi, K.; Akiyama, S.; Yoshimura, T. Multilayer formation using oppositely charged gold- and silver- dendrimer nanocomposites. Langmuir 2003, 19, 76779–77681. [Google Scholar]
- Hou, F.; Xu, H.; Zhang, L.; Fu, Y.; Wang, Z.; Zhang, X. Hydrigen-binding based multilayer assemblies by self–deposition of dendrimer. Chem. Commun. 2003, 874–875. [Google Scholar]
- Zhang, H.; Fu, Y.; Wang, D.; Wang, L.; Wang, Z.; Zhang, X. Hydrogen-bonding-directed layer-by-layer assembly of dendrimer and poly(4-vinylpyridine) and micropore formation by post-base treatment. Langmuir 2003, 19, 8497–8502. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Gao, J.; Wang, Z. Control of composition in the multilayer films fabricated from mixed solutions containing two dendrimers. J. Colloid Interface Sci. 2005, 287, 207–212. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J. pH-sensitive thin films composed of poly(methacrylic acid) and carboxyl-terminated dendrimer. Sens. Lett. 2008, 6, 250–252. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J. Layer-by-layer assembled thin films composed of carboxyl-terminated poly(amidoamine) dendriemr as a pH-sensitive nano-device. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J. pH-Stability of layer-by-layer thin films composed of carboxyl-terminated poly(amidoamine) dendrimer and poly(acrylic acid). Kobunshi Ronbunshu 2009, 66, 75–78. [Google Scholar] [CrossRef]
- Ito, M.; Imae, T. Self-assembled monolayer of carboxyl-terminated poly(amido amine) dendrimer. J. Nansci. Nanotechnol. 2006, 6, 1667–1672. [Google Scholar]
- Liu, Y.; Bruening, M.L.; Bergbreiter, D.E.; Crooks, R.M. Multilayer dendrimer-polyanhydride composite films on glass, silicone, and gold wafers. Angew. Chem. Int. Ed. 1997, 36, 2114–2116. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Y.; Crooks, R.M.; Bergbreiter, D.E. Preparation of h8ighly impermeable hyperbranched polymer thin-film coatings using dendrimers first as building blocks and then as in situ thermosetting agents. J. Am. Chem. Soc. 1999, 121, 923–930. [Google Scholar]
- Yoon, H.C.; Kim, H.-S. Multilayered assembly of dendrimers with enzymes on gold: Thickness-controlled biosensing interface. Anal. Chem. 2000, 72, 922–926. [Google Scholar] [CrossRef]
- Yoon, H.C.; Hong, M.-Y.; Kim, H.-S. Functionalization of a poly(amidoamine) dendrimer with ferrocenyls and its application to the construction of a reagentless enzyme electrode. Anal. Chem. 2000, 72, 4420–4427. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Jia, X.; Cao, W.; Li, M. Self-assembly ultrathin films based on dendrimers. Chem. Commun. 2000, 511–512. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Jia, X.; Li, Y.; Qin, Y.; Chen, J.; Zhao, X.-S.; Cao, W.; Li, M.; Wei, Y. Fabrication of covalently attached ultrathin films based on dendrimers via H-binding attraction and subsequent UV irradiation. Macromol. Rapid Commun. 2001, 22, 583–586. [Google Scholar] [CrossRef]
- Wang, J.; Jia, X.; Zhong, H.; Luo, Y.; Zhao, X.; Cao, W.; Li, M. Self-assembled multilayer films based on dendrimers with covalent interlayer linkage. Chem. Mater. 2002, 14, 2854–2858. [Google Scholar]
- Watanabe, S.; Regen, S.L. Dendrimers as building blocks for multilayer construction. J. Am. Chem. Soc. 1994, 116, 8855–8856. [Google Scholar] [CrossRef]
- Anzai, L.; Nishimura, M. Layer-by-layer deposition of avidin and polymers on a solid surface to prepare thin films: significant effects of molecular geometry of the polymers on the deposition behaviour. J. Chem. Soc. Perkin Trans. 2 1997, 1887–1889. [Google Scholar] [CrossRef]
- Anzai, J.; Kobayashi, Y.; Nakamura, N.; Nishimura, M.; Hoshi, T. Layer-by-layer construction of multilayer thin films composed of avidin and biotin–labeled poly(amine)s. Langmuir 1999, 15, 221–226. [Google Scholar]
- Wilchek, M.; Bayer, E.A. The avidin-biotin complex in bioanalytical applications. Anal. Biochem. 1988, 171, 1–32. [Google Scholar] [CrossRef]
- Cassier, T.; Lowack, K.; Decher, G. Layer-by-layer assembled protein/polymer hybrid films: nanoconstruction via specific recognition. Supramol. Sci. 1998, 5, 309–315. [Google Scholar] [CrossRef]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Redox labelled avidin for enzyme sensor architectures. Biosens. Bioelectron. 2003, 19, 239–247. [Google Scholar] [CrossRef]
- Inoue, H.; Sato, K.; Anzai, J. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin–labeled poly(ethyleneimine) and avidin. Biomacromolecules 2005, 6, 27–29. [Google Scholar] [CrossRef]
- Sato, K.; Kodama, D.; Naka, Y.; Anzai, J. Electrochemically induced disintegration of layer-by-layer assembled thin films composed of 2–iminobiotin–labeled poly(ethyleneimine) and avidin. Biomacromolecules 2006, 7, 3302–3305. [Google Scholar] [CrossRef]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Yang, Y.; Huang, J.; Zhao, Z.; Xu, X.; Anzai, J.; Osa, T.; Chen, Q. Amperometric choline biosensors prepared by layer–by–layer deposition of choline oxidase on the Prussian blue-modified platinum electrode. Talanta 2006, 70, 852–858. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.-J.; Chen, H.-Y. Electrochemical biosensors based on layer–by–layer assemblies. Electroanalysis 2006, 18, 1737–1748. [Google Scholar] [CrossRef]
- Siqueira, J.R., Jr.; Caseli, L.; Crespilho, F.N.; Zucolotto, V.; Oliveira, O.N., Jr. Immobilization of biomolecules on nanostructured films for biosensing. Biosens. Bioelectron. 2010, 23, 1254–1263. [Google Scholar]
- Huang, J.; Yang, Y.; Shi, H.; Song, Z.; Zhao, Z.; Anzai, J.; Osa, T.; Chen, Q. Multi-walled carbon nanotubes-based glucose biosensor prepared by a layer–by–layer technique. Mater. Sci. Eng. C 2006, 26, 113–117. [Google Scholar] [CrossRef]
- Mark, S.S.; Sandhyarani, N.; Zhu, C.; Campagnolo, C.; Batt, C.A. Dendrimer-functionalized self-assembled monolayers as a surface plasmon resonance sensor surface. Langmuir 2004, 20, 6808–6817. [Google Scholar] [CrossRef]
- Sihgh, P.; Onodera, T.; Mizuta, Y.; Matsumono, K.; Miura, N.; Toko, K. Dendrimer modified biochip for detection of 2,3,6-trinitrotoluene on SPR immunosensor: Fabrication and advantages. Sens. Actuators B 2009, 137, 403–409. [Google Scholar] [CrossRef]
- Feng, C.L.; Yin, M.; Zhang, D.; Zhu, S.; Caminade, A.M.; Majoral, J.P.; Müllen, K. Fluorescent core–shell star polymers based biosensors for ultrasensitive DNA detection by surface plasmon fluorescence spectroscopy. Macromol. Rapid Commun. 2011, 32, 679–683. [Google Scholar] [CrossRef]
- Qian, L.; Liu, Y.; Song, Y.; Li, Z.; Yang, X. Electrodeposition of Pt nanoclusters on the surface modified by monolayer poly(amidoamine) dendrimer film. Electrochem. Commun. 2005, 7, 1209–1212. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, W.; Wang, J.; Yang, C.; Yang, F.; Yang, X. A sensitive impedimetric thrombin aptasensor based on polyamidoamine dendrimer. Talanta 2009, 78, 1240–1245. [Google Scholar] [CrossRef]
- Suk, J.; Lee, J.; Kwak, J. Electrochemistry on alternate structures of gold nanoparticles and ferrocene-tethered polyamidoamine dendrimers. Bull. Korean Chem. Soc. 2004, 25, 1681–1686. [Google Scholar] [CrossRef]
- Lojou, E.; Bianco, P. Assemblies of dendrimers and proteins on carbon and gold electrodes. Bioelectrochem. 2006, 69, 237–247. [Google Scholar] [CrossRef]
- Hodak, J.; Etchenique, R.; Calvo, E.J. Layer-by-layer self-assembly of glucose oxidase with a poly(allylamine)ferrocene redox mediator. Langmuir 1997, 13, 2708–2716. [Google Scholar] [CrossRef]
- Anzai, J.; Kobayashi, Y. Construction of multilayer thin films of enzymes by means of sugar-lectin interactions. Langmuir 2000, 16, 2851–2856. [Google Scholar] [CrossRef]
- Zucolotto, V.; Pinto, A.P.A.; Tumolo, T.; Moreas, M.L.; Baptista, M.S.; Riul, A., Jr.; Araujo, A.P.U.; Oliveira, O.N., Jr. Catechol biosensing using a nanostructured layer-by-layer film containing Cl-catechol 1,2-dioxygenase. Biosens. Bioelectron. 2006, 21, 1320–1326. [Google Scholar] [CrossRef]
- He, P.; Li, M.; Hu, N. Interaction of heme proteins with poly(propyleneimine) dendrimers in layer-by-layer assembly films under different pH conditions. Biopolymers 2005, 79, 310–323. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, N. Assembly of myoglobin layer-by-layer films with poly(propyleneimine) dendrimer-stabilized gold nanoparticles and its application in electrochemical biosensing. Biosens. Bioelectron. 2007, 23, 393–399. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Y.; Yang, X.; Li, C. Photoelectrochemical glucose biosensor incorporating CdS nanoparticles. Particuology 2009, 7, 347–352. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, H.; Yang, X.; Xu, L.; Li, C. Sensitive biosensors based on (dendrimer encapsulated Pt nanoparticles)/enzyme multilayers. Electroanalysis 2007, 19, 698–703. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, Q.; Xiao, F.; Shi, G.; Jin, L. Layer-by-layer self-assembled acetylcholineesterase/PAMAM-Au on CNTs modified electrode for sensing pesticides. Bioelectrochemistry 2010, 77, 139–144. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, Y.; Yang, X.; Li, C. An enhanced biosensor for glutamate based on self-assembled carbon nanotubes and dendrimer-encapsulated platinum nanobiocomposites–doped polypyrrole film. Anal. Chim. Acta 2007, 597, 145–150. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, Y.; Xu, L.; Yang, X.; Li, C. Amperometric glutamate biosensor based on self-assembling glutamate dehydrogenase and dendrimer–encapsulated platinum nanoparticles onto carbon nanotubes. Talanta 2007, 73, 438–443. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Ghica, M.E.; Zucolotto, V.; Nart, F.C.; Oliveira, O.N., Jr.; Brett, C.M.A. Electroactive nanostructured membranes (ENM): Synthesis and electrochemical properties of redox mediator-modified gold nanoparticles using a dendrimer layer-by-layer approach. Electroanalysis 2007, 19, 805–812. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Ghica, M.E.; Gouveia-Caridale, C.; Oliveira, O.N., Jr.; Brett, C.M.A. Enzyme immobilization on electroactive nanostructured membranes (ENM): Optimised architectures for biosensing. Talanta 2008, 76, 922–928. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Ghica, M.E.; Florescu, M.; Nart, F.C.; Oliveira, O.N., Jr.; Brett, C.M.A. A strategy for enzyme immobilization on layer-by-layer dendriemer-gold nanoparticle electrocatalytic membrane incorporating redox mediator. Electrochem. Commun. 2006, 8, 1665–1670. [Google Scholar] [CrossRef]
- Siqueira, J.R., Jr.; Abouzar, M.H.; Poghossian, A.; Zucolotto, V.; Oliveira, O.N., Jr.; Schöning, M.J. Penicillin biosensor based on a capacitive field-effect structure functionalized with a dendrimer/carbon nanotube multilayer. Biosens. Bioelectron. 2009, 25, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.R., Jr.; Werner, C.F.; Bäcker, M.; Poghossian, A.; Zucolotto, V.; Oliveira, O.N., Jr.; Schöning, M.J. Layer-by-layer assembly of carbon nanotubes incorporated in light-addresable potentiometric sensors. J. Phys. Chem. C 2009, 113, 14765–14770. [Google Scholar] [CrossRef]
- Fernandes, E.G.R.; Vieira, N.C.S.; de Queiroz, A.A.A.; Guimarães, F.E.G.; Zucolotto, V. Immobilization of poly(propylene imine) dendrimer/nickel phthalocyanine as nanostructured multilayer films to be used as gate membranes for SEGFET pH sensors. J. Phys. Chem. C 2010, 114, 6478–6483. [Google Scholar]
- Centurion, L.M.P.C.; Moreira, W.C.; Zucolotto, V. Tailoring molecular architectures with cobalt tetrasulfonated phthalocyanine: Immobilization in layer-by-layer films and sensing applications. J. Nanosci. Nanotechnol. 2012, 12, 2399–2405. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Figueiredo, A.; Queiroz, A.A.A.; Zucolotto, V.; Guimarãez, F.E.G. Self-assembled films of dendrimers and metallophthalocyanines as FET–based glucose biosensors. Sensors 2011, 11, 9442–9449. [Google Scholar] [CrossRef] [Green Version]
- der Mercato, L.L.; Rivera–Gil, P.; Abbasi, A.Z.; Ochs, M.; Ganas, C.; Zins, I.; Sonnichsen, C.; Parak, W.J. LbL multilayer capsules: recent progress and future outlook for their use in life sciences. Nanoscale 2010, 2, 458–467. [Google Scholar] [CrossRef]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Delv. Rev. 2011, 63, 809–821. [Google Scholar] [CrossRef]
- Ariga, K.; McShane, M.; Lvov, Y.M.; Ji, Q.; Hill, J.P. Layer-by-layer assembly for drug delivery and related applications. Exp. Opin. Drug Deliv. 2011, 8, 633–644. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef]
- Khopade, A.J.; Caruso, F. Electrostatically assembled polyelectrolyte/dendrimer multilayer films as ultrathin nanoreservoirs. Nano Lett. 2002, 2, 415–418. [Google Scholar] [CrossRef]
- Moraes, M.L.; Baptista, M.S.; Itri, R.; Zucolotto, V.; Oliveira, O.N., Jr. Immobilization of liposomes in nanostructured layer–by–layer films containing dendrimers. Mater. Sci. Eng. C 2008, 28, 467–471. [Google Scholar] [CrossRef]
- Geraldo, V.P.N.; Moraes, M.L.; Zucolotto, V.; Oliveira, O.N., Jr. Immobilisation of ibuprofen-containing nanospheres in layer-by-layer films. J. Nanosci. Nanotechnol. 2011, 11, 1167–1174. [Google Scholar] [CrossRef]
- Gui, Z.; Qian, J.; An, Q.; Zhao, Q.; Jin, H.; Du, B. Layer-by-layer self-assembly, controllable disintegration of polycarboxybetaine multilayers and preparation of free-standing films at physiological conditions. J. Mater. Chem. 2010, 20, 1467–1474. [Google Scholar] [CrossRef]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally modulated insulin release from microgel thin films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar]
- Sato, H.; Takano, Y.; Sato, K.; Anzai, J. Electrochemically controlled release of α,β,γ,δ-tetrakis (4-N-methylpyridyl)porphine from layer-by-layer thin films. J. Colloid Interface Sci. 2009, 333, 141–144. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Bai, S.; Huo, F.; Wang, Z.; Zhang, X. Base-induced release of molecules from hydrogen bonding directed layer-by-layer film. Chin. J. Polym. Sci. 2003, 21, 499–503. [Google Scholar]
- Khopade, A.J.; Caruso, F. Stepwise self-assembled poly(amidoamine) dendrimer and poly(styrenesulfonate) microcapsules as sustained delivery vehicles. Biomacromolecules 2002, 3, 1154–1162. [Google Scholar] [CrossRef]
- Levedeva, O.V.; Kim, B.-S.; Gröhn, F.; Vinogradova, O.I. Dendrimer-encapsulated gold nanoparticles as building blocks for multilayer microshells. Polymer 2007, 48, 5024–5029. [Google Scholar] [CrossRef]
- Kim, B.-S.; Lebedeva, O.V.; Kim, D.H.; Caminade, A.-M.; Majoral, J.-P.; Knoll, W.; Vinogradova, O.I. Assembly and mechanical properties of phosphorus dendrimer/polyelectrolyte multilayer microcapsules. Langmuir 2005, 21, 7200–7206. [Google Scholar]
- Kim, B.-S.; Lebedeva, O.V.; Koynov, K.; Gong, H.; Caminade, A.-M.; Majoral, J.-P.; Vinogradova, O.I. Effect of dendrimer generation on the assembly and mechanical properties of DNA/phosphorus dendrimer multilayer microcapsules. Macromolecules 2006, 39, 5479–5483. [Google Scholar]
- Kim, B.-S.; Lebedeva, O.V.; Park, M.-K.; Knoll, W.; Caminade, A.-M.; Majoral, J.-P.; Vinogradova, O.I. THF-induced stiffening of polyelectrolyte/phosphorus dendrimer multilayer microcapsules. Polymer 2010, 51, 4525–4529. [Google Scholar] [CrossRef]
- Feng, C.-L.; Caminade, A.-M.; Majoral, J.-P.; Zhang, D. Selective encapsulation of dye molecules in dendrimer/polymer multilayer microcapsules by DNA hybridization. J. Mater. Chem. 2010, 20, 1438–1441. [Google Scholar] [CrossRef]
- Feng, C.-L.; Caminade, A.-M.; Majoral, J.-P.; Gu, J.; Zhu, S.; Su, H.; Hu, X.; Zhang, D. DNA hybridization induced selective encapsulation of small dye molecules in dendrimer based microcapsules. Analyst 2010, 135, 2939–2944. [Google Scholar] [CrossRef]
- Endo, Y.; Sato, K.; Anzai, J. Preparation of avidin-containing polyelectrolyte microcapsules and their uptake and release properties. Polym. Bull. 2011, 66, 711–720. [Google Scholar] [CrossRef]
- Endo, Y.; Sato, K.; Yoshida, K.; Anzai, J. Avidin/PSS membrane microcapsules with biotin-binding activity. J. Colloid Interface Sci. 2011, 360, 519–524. [Google Scholar] [CrossRef]
- Strydom, S.J.; Rose, W.E.; Otto, D.P.; Liebenberg, W.; de Villiers, M.M. Poly(amidoamine) dendrimer-mediated synthesis and stabilization of silver sulfonamide nanoparticles with increased antibacterial activity. Nanomedicine 2013, 9, 85–93. [Google Scholar] [CrossRef]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amono and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef]
- Polcyn, P.; Jurczak, M.; Rajnisz, A.; Solecka, J.; Urbanczyk-Lipkowska, Z. Design of antimicrobially active small amphiphilic peptide dendrimers. Molecules 2009, 14, 3881–3905. [Google Scholar] [CrossRef]
- Janiszewska, J.; Sowińska, M.; Rajinisz, A.; Solecka, J.; Łącka, I.; Milewski, S.; Urbanczyk-Lipkowska, Z. Novel dendrimeric lipopeptides with antifungal activity. Bioorg. Med. Chem. Lett. 2012, 22, 1388–1393. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J. Preparation of dendrimer-loaded microcapsules by a layer-by-layer deposition of polyelectrolytes. Mater. Sci. Eng. C 2009, 29, 2024–2028. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.H.; Swanson, S.D.; Ge, S.; Cao, Z.; Van Antwerp, M.E.; Landmark, K.L.; Baker, J.R., Jr. Dendrimer-functionalized shell-crosslinked iron oxide nanoparticles for in-vivo magnetic resonance imaging of tumors. Adv. Mater. 2008, 20, 1671–1678. [Google Scholar] [CrossRef]
- Wang, S.H.; Shi, X.; Van Antwerp, M.; Cao, Z.; Swanson, S.D.; Bi, X.; Baker, J.R., Jr. Dendrimer-functionalized iron oxide nanoparticles for specific targeting and imaging of cancer cells. Adv. Funct. Mater. 2007, 17, 3043–3050. [Google Scholar] [CrossRef]
- Khopade, A.J.; Caruso, F. Surface-modification of polyelectrolyte multilayer-coated particles for biological applications. Langmuir 2003, 19, 6219–6225. [Google Scholar] [CrossRef]
- Hernandez-Lopez, J.-L.; Khor, H.L.; Caminade, A.-M.; Majoral, J.-P.; Mittler, S.; Knoll, W.; Kim, D.H. Bioactive multilayer thin films of charged N,N-disubstituted hydrazine phosphorus dendrimers fabricated by layer-by-layer self-assembly. Thin Solid Films 2008, 516, 1256–1264. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, K.; Anzai, J.-i. Dendrimers in Layer-by-Layer Assemblies: Synthesis and Applications. Molecules 2013, 18, 8440-8460. https://doi.org/10.3390/molecules18078440
Sato K, Anzai J-i. Dendrimers in Layer-by-Layer Assemblies: Synthesis and Applications. Molecules. 2013; 18(7):8440-8460. https://doi.org/10.3390/molecules18078440
Chicago/Turabian StyleSato, Katsuhiko, and Jun-ichi Anzai. 2013. "Dendrimers in Layer-by-Layer Assemblies: Synthesis and Applications" Molecules 18, no. 7: 8440-8460. https://doi.org/10.3390/molecules18078440