Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene
Abstract
:1. Introduction
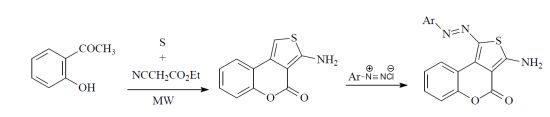
2. Results and Discussion

| Dye No | Color on polyester (2% shade) | L* | a* | b* | C* | h* | K/S |
|---|---|---|---|---|---|---|---|
| 7a | Orange | 58.12 | 52.91 | 64.18 | 83.18 | 50.50 | 20.66 |
| 7b | Brown | 59.74 | 36.18 | 42.69 | 55.96 | 49.71 | 8.55 |
| 7c | Dark orange | 46.13 | 48.90 | 51.72 | 71.18 | 46.60 | 28.68 |
| 7d | Orange | 59.26 | 52.96 | 49.46 | 72.46 | 43.04 | 11.86 |
| 7e | Violet | 39.61 | 44.29 | −11.47 | 45.75 | 345.48 | 8.87 |
| DyeNo | λmax(DMF) | Wash fastness | Perspiration fastness | Light fastness | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline | Acidic | ||||||||||
| Alt | SC | SW | Alt | SC | SW | Alt | SC | SW | |||
| 7a | 490 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 |
| 7b | 491 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 2 |
| 7c | 497 | 5 | 4–5 | 4–5 | 5 | 4–5 | 5 | 5 | 4–5 | 5 | 2 |
| 7d | 500 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 2–3 |
| 7e | 662 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 |
3. Experimental
3.1. General
3.2. Reaction of Compound 4 with Aromatic Diazonium Salts
3.3. High Temperature Dyeing Method (HT)
3.3.1. Materials
3.3.2. Dyeing
3.4. Color Measurements and Analyses
3.4.1. Color Measurements
3.4.2. Fastness Testing
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gewald, K.; Schinke, E.; Boettcher, H. Heterocycles from CH-acidic nitriles. VIII. 2-Aminothiophenes from methylene-active nitriles, carbonyl compounds, and sulfur. Chem. Ber. 1966, 99, 94–100. [Google Scholar] [CrossRef]
- Rangnegar, D.W.; Sabnis, R.W. Novel substituted 2-acetamido thiophene-4-styryl disperse dyes. J. Chem. Tech. Bio. 1993, 56, 401–405. [Google Scholar]
- Hallas, G.; Choi, J.-H. Synthesis and spectral properties of azo dyes derived from 2-aminothiophenes and 2-aminothiazoles. Dyes Pigm. 1999, 42, 249–265. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Afifi, T.H. Novel azo disperse dyes derived from aminothiophenes: Synthesis and UV-visible studies. Dyes Pigm. 2006, 70, 8–17. [Google Scholar] [CrossRef]
- Fondjo, E.S.; Tsemeugne, J.; Sondengam, B.L.; Oppenlaender, T.; Wabo, H.K.; Tane, P.; Connolly, J.D.; Dehaen, W.; Rohand, T.; Kikuchi, H.; Oshima, Y. Coupling of Two Diazotized 3-Aminothieno[3,4-c]coumarins with Aromatic Amines. J. Heterocycl. Chem. 2011, 48, 1295–1301. [Google Scholar] [CrossRef]
- Yen, M.S.; Wang, I.J. Synthesis and absorption spectra of hetarylazo dyes derived from coupler 4-aryl-3-cyano-2-minothiophenes. Dyes Pigm. 2004, 61, 243–250. [Google Scholar] [CrossRef]
- Hallas, G.; Cho, J.-H. Synthesis and properties of novel aziridinyl azo dyes from 2-aminothiophenes-Part 2: Application of some disperse dyes to polyester. Dyes Pigm. 1999, 40, 119–129. [Google Scholar] [CrossRef]
- Bhattia, H.S.; Seshadri, S. Novel disperse dyes from benzo[b]thiophene-3(2H)-one-1,1-dioxide and ethyl benzo[b]thien-3(2H)-ylidenecyanoacetate,S,S-dioxide: Synthesis and properties. Dyes Pigm. 2004, 62, 83–92. [Google Scholar] [CrossRef]
- Maradiya, H.R.; Patel, V.S. Thiophene based monoazo disperse dyes for polyester fabric. J. Serb. Chem. Soc. 2002, 67, 17–25. [Google Scholar] [CrossRef]
- Rodighiero, P.; Pastorini, G.; Dalla Via, L.; Gia, O.; Magno, S.M. Synthesis and biological activity of linear and angular 4-methoxymethylthienocoumarins and 4-acetoxymethylthienocoumarins. Farmaco 1998, 53, 313–319. [Google Scholar] [CrossRef]
- Manna, F.; Chimenti, F.; Bolasco, A.; Cenicola, M.L.; D'Amico, M.; Parrillo, C.; Rossi, F.; Marmo, E. Antiinflammatory, analgesic and antipyretic N-acetyl-Δ2-pyrazolines and dihydrothienocoumarins. Eur. J. Med. Chem. 1992, 27, 633–639. [Google Scholar] [CrossRef]
- Al-Saleh, B.; El-Apasery, M.A. Studies with condensed aminothiophenes: Further investigation on the reactivity of condensed aminothiophenes toward electron poor olefins and acetylenes under microwave heating. J. Het. Chem. 2006, 43, 559–564. [Google Scholar] [CrossRef]
- Abdelkhalik, M.M.; Negm, A.M.; Elkhouly, A.I.; Elnagdi, M.H. Studies with condensed aminothiophenes: Further investigation of reactivity of aminothienocoumarines and aminothienobenzo[h]coumarines toward electron-poor olefins and acetylenes. Heteroat. Chem. 2004, 15, 502–507. [Google Scholar] [CrossRef]
- Al-Saleh, B.; Abdelkhalik, M.M.; El-Apasery, M.A.; Elnagdi, M.H. Studies with condensed thiophenes: Reactivity of condensed aminothiophenes toward carbon and nitrogen electrophiles. J. Chem. Res. 2005, 2005, 23–26. [Google Scholar] [CrossRef]
- Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. Microwave-induced organic reaction enhancement chemistry. 2. Simplified techniques. J. Org. Chem. 1991, 56, 6968–6970. [Google Scholar]
- Fondjo, E.S.; Doepp, D.; Henkel, G. Reactions of some anellated 2-aminothiophenes with electron poor acetylenes. Tetrahedron 2006, 62, 7121–7131. [Google Scholar] [CrossRef]
- Chrysler, L.P. Methods of Test for Color Fastness of Textiles and Leather, 7th ed.; Bradford: London, UK, 1990; pp. 89–94. [Google Scholar]
- Sample Availability:Samples of compounds 4 and 7a–e are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Mousawi, S.M.; El-Apasery, M.A. Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene. Molecules 2013, 18, 8837-8844. https://doi.org/10.3390/molecules18088837
Al-Mousawi SM, El-Apasery MA. Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene. Molecules. 2013; 18(8):8837-8844. https://doi.org/10.3390/molecules18088837
Chicago/Turabian StyleAl-Mousawi, Saleh Mohammed, and Morsy Ahmed El-Apasery. 2013. "Synthesis of Some Monoazo Disperse Dyes Derived from Aminothienochromene" Molecules 18, no. 8: 8837-8844. https://doi.org/10.3390/molecules18088837