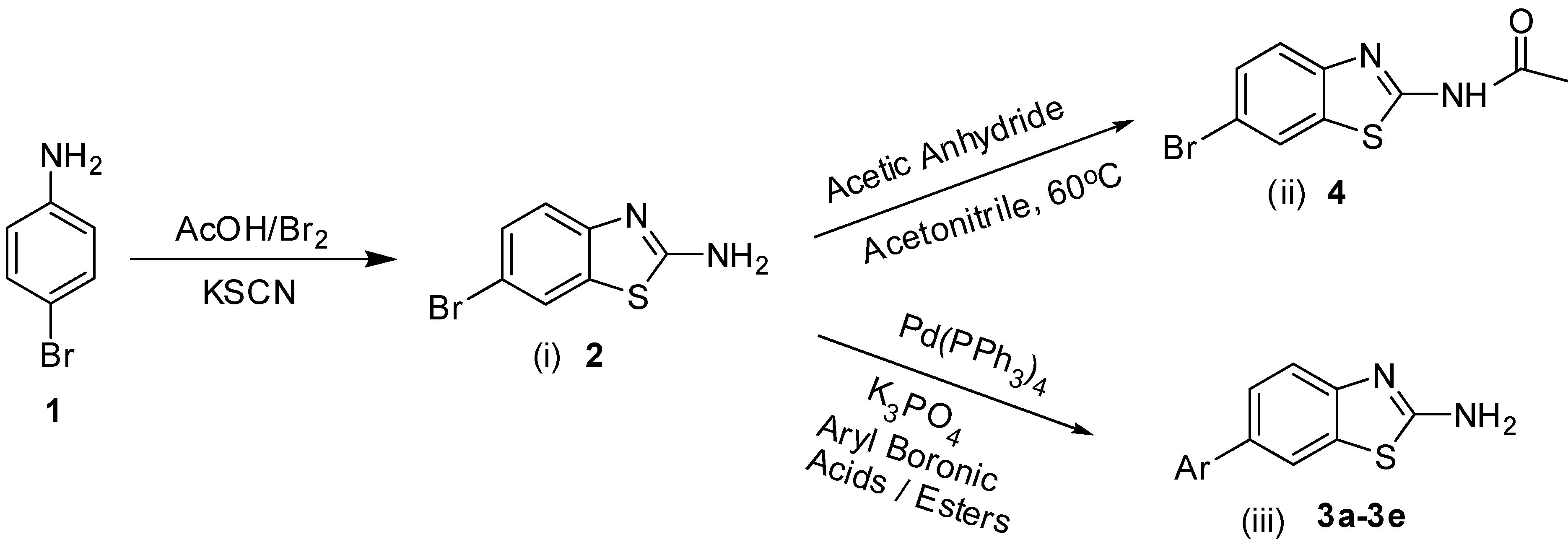
Efficient Synthesis of 2-Amino-6-Arylbenzothiazoles via Pd(0) Suzuki Cross Coupling Reactions: Potent Urease Enzyme Inhibition and Nitric Oxide Scavenging Activities of the Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Entry | Aryl Boronic Acid/Aryl Boronic Pinacol Ester | Product | Solvent/H2O (4:1) | Yield% [a] |
|---|---|---|---|---|
| 1 |  |  3a | Toluene | 65 |
| 2 | 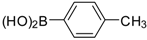 | 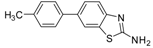 3a | Dioxane | 75 |
| 3 |  |  3b | Toluene | 61 |
| 4 | 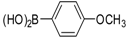 | 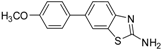 3c | DMF | 64 |
| 5 |  | 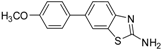 3c | Dioxane | 71 |
| 6 |  | 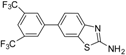 3d | Dioxane | 67 |
| 7 | 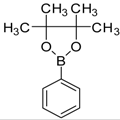 |  3e | Dioxane | 78 |
2.2. Pharmacology
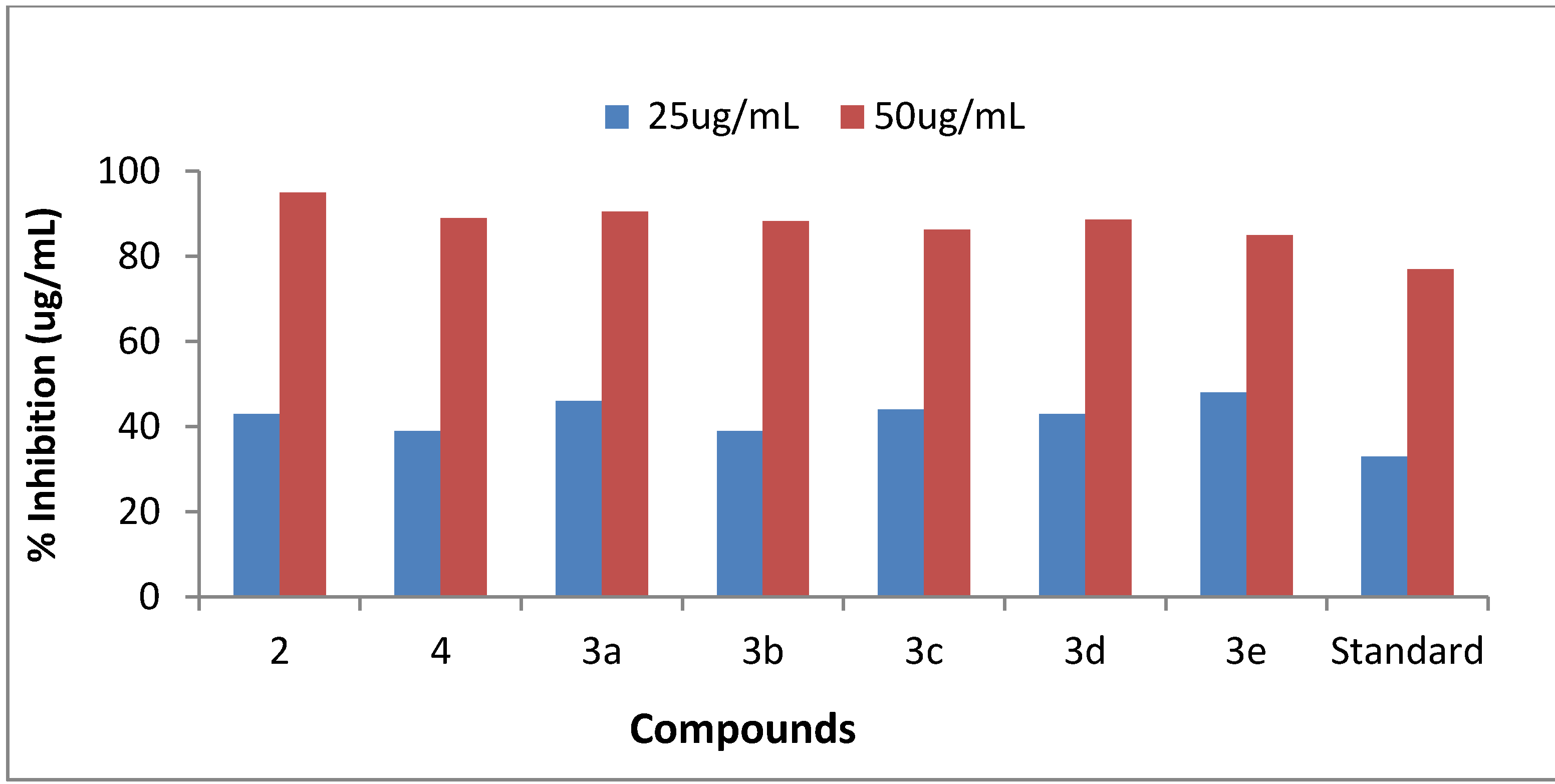
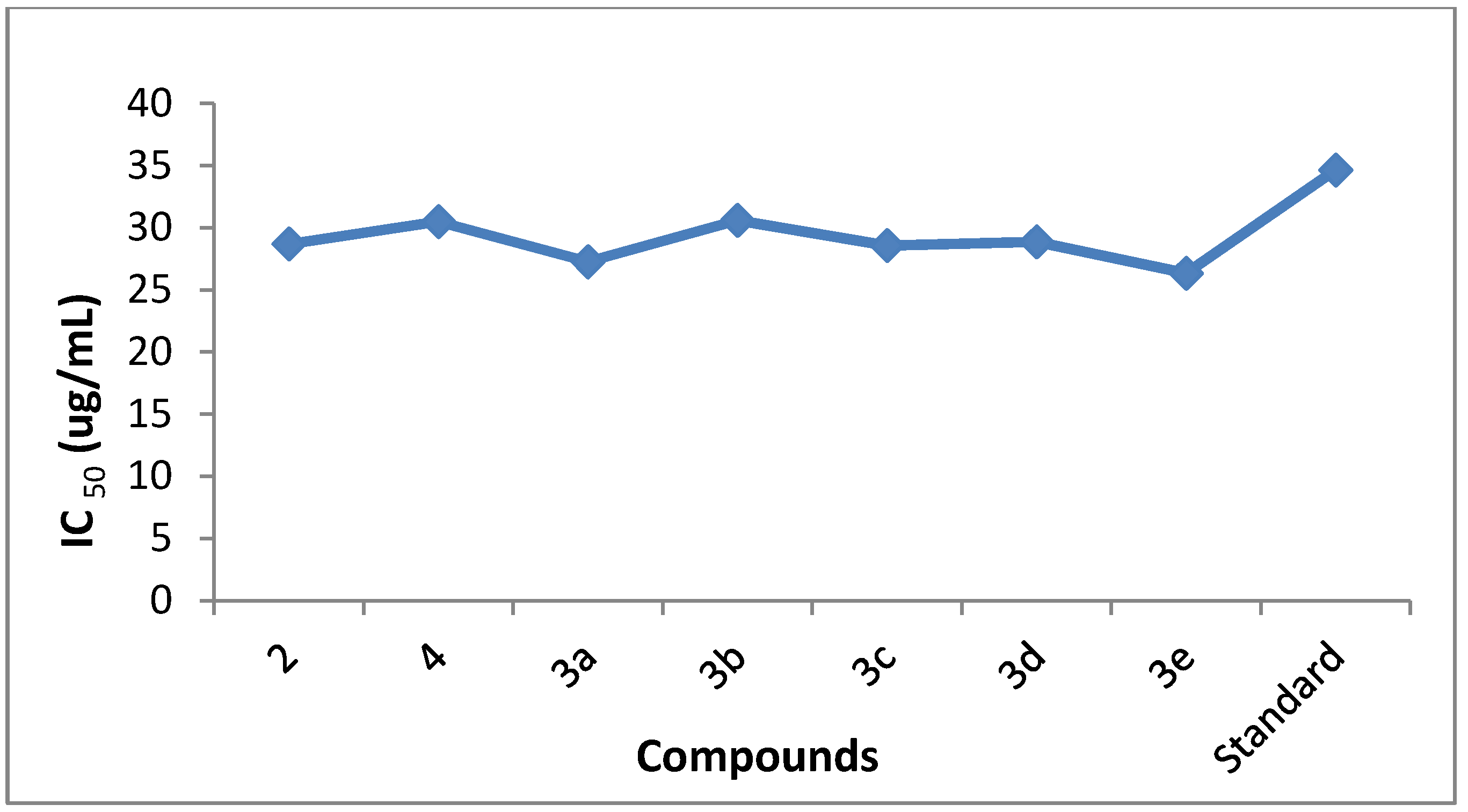
2.2.1. Antiurease Activity
| Compound | Percentage inhibition at 25 µg/mL | Percentage inhibition at 50 µg/mL | IC50 (µg/mL) |
|---|---|---|---|
| 2 | 43 ± 0.0034 | 95 ± 0.005 | 28.4 |
| 4 | 39 ± 0.001 | 89 ± 0.009 | 30.5 |
| 3a | 46 ± 0.005 | 90.49 ± 0.002 | 27.27 |
| 3b | 39 ± 0.0012 | 88.19 ± 0.007 | 30.6 |
| 3c | 44 ± 0.005 | 86.24 ± 0.001 | 28.57 |
| 3d | 43 ± 0.007 | 88.6 ± 0.002 | 28.88 |
| 3e | 48 ± 0.005 | 85 ± 0.002 | 26.35 |
| Standard | 33 ± 0.04 | 77 ± 0.01 | 34.65 |
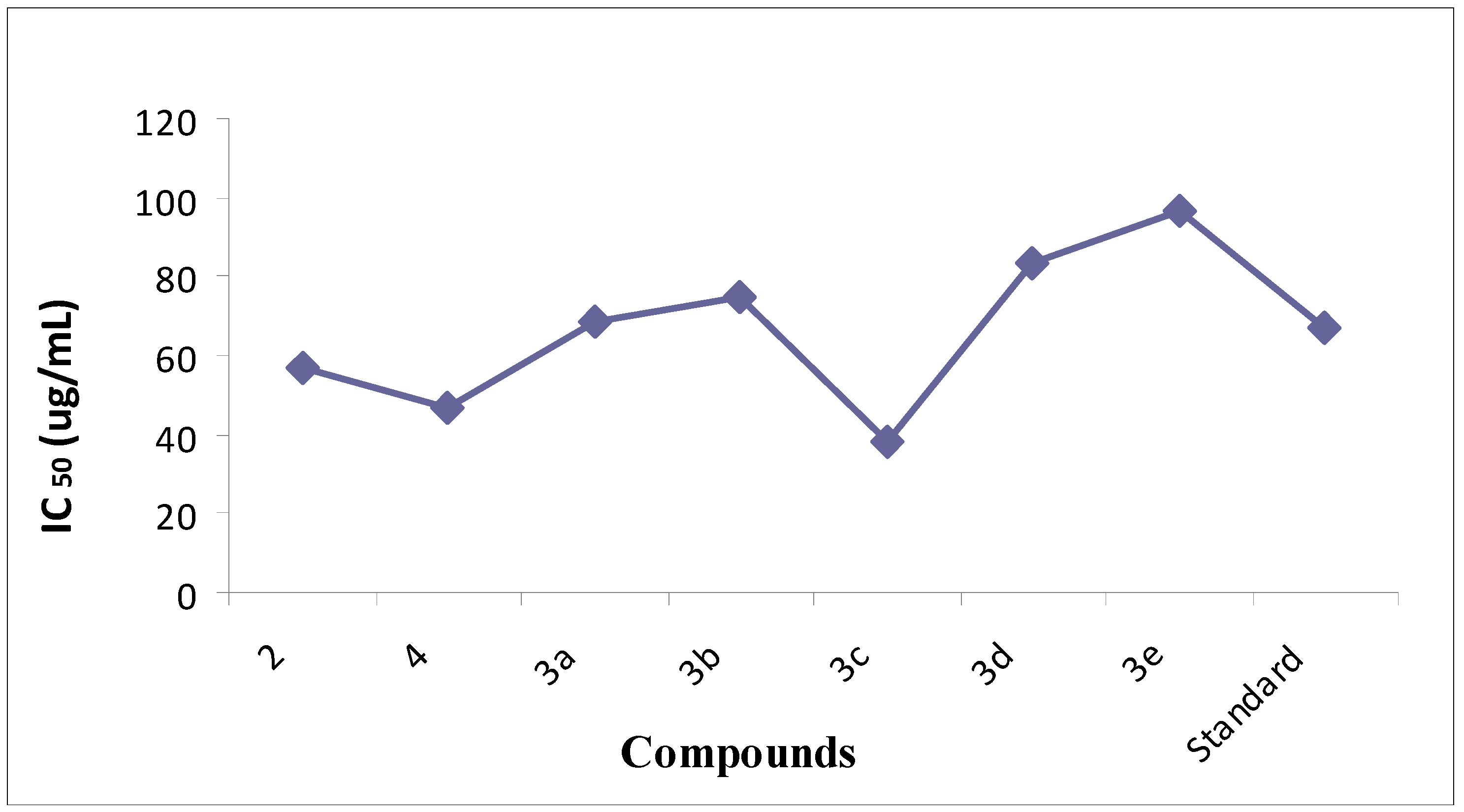
2.2.2. Nitric Acid (NO) Scavenging Percentage (Antioxidant Activity)
| Compound | Nitric Oxide Percentage Scavenging Activity at 25 µg/mL | Nitric Oxide Percentage Scavenging Activity at 50 µg/mL | Nitric Oxide Percentage Scavenging Activity at 100 µg/mL | IC50 (µg/mL) |
|---|---|---|---|---|
| 1 2 | 20 ± 0.004 | 48 ± 0.0007 | 90 ± 0.0007 | 57.8 |
| 2 4 | 25 ± 0.001 | 54 ± 0.002 | 92 ± 0.002 | 46.5 |
| 3 3a | 24 ± 0.003 | 56 ± 0.001 | 95 ± 0.001 | 68.3 |
| 4 3b | 17 ± 0.006 | 34 ± 0.005 | 66 ± 0.005 | 75 |
| 5 3c | 31 ± 0.022 | 67 ± 0.002 | 98 ± 0.002 | 38.19 |
| 6 3d | 10 ± 0.01 | 23 ± 0.009 | 63 ± 0.009 | 83.75 |
| 7 3e | 9 ± 0.007 | 22 ± 0.001 | 52 ± 0.001 | 96.66 |
| Standard | 40.5 ± 0.01 | 70.5 ± 0.91 | 99.5 ± 0.91 | 66.66 |

3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of 2-Amino-6-arylbenzothiazoles
3.3. Preparation of N-(6-bromobenzo[d]thiazol-2-yl)acetamide (4)
3.4. Characterization Data
3.5. NO Scavenging Activity
3.6. Antiurease Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Paramashivappa, R.; Phani, K.P.; Rao, P.V.S. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Biorg. Med.Chem. Lett. 2003, 13, 657–660. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Sing, I.; Saxena, K.K.; Kumar, A. Synthesis, Characterization and biological activity of various substituted benzothiazole derivatives. Dig. J. Nanomater. Bios. 2010, 5, 67–76. [Google Scholar]
- Suvarna, K.; Swain, S.P.; Gandhi, A.M. Synthesis and evaluation of novel benzothiazole derivatives against human cervical cancer cell lines. Ind. J. Pharm. Sci. 2007, 69, 46–50. [Google Scholar] [CrossRef]
- Rajeeva, B.; Srinivasulu, N.; Shantakumar, S.M. Synthesis and antimicrobial activity of some new 2-substituted benzothiazole derivatives. E-J. Chem. 2009, 6, 775–779. [Google Scholar] [CrossRef]
- Giorgioni, G.; Accorroni, B.; Stefano, A.D.; Marucci, G.; Siniscalchi, A.; Claudi, F. Benzimidazole, Benzoxazole and Benzothiazole Derivatives as 5HT2B Receptor Ligands. Synthesis and Preliminary Pharmacological Evaluation. Med. Chem. Res. 2005, 14, 57–73. [Google Scholar] [CrossRef]
- Mahran, M.A.; William, S.; Ramzy, F.; Sembel, A.M. Synthesis and in vitro evaluation of new benzothiazole derivatives as schistosomicidal agents. Molecules. 2007, 12, 622–633. [Google Scholar] [CrossRef]
- Tamm, I.; Folker, K.; Clifford, H.S.; Dorothea, H.; Horsfall, L.F. Inhibition of influenza virus multiplication by N-glycosides of Benzimidazoles. J. Exp. Med. 1954, 99, 227–250. [Google Scholar] [CrossRef]
- Yadav, P.S.; Devprakash; Senthilkumar, G.P. Benzothiazole: Different methods of synthesis and diverse biological activities. Int. J. Pharm. Sci. Drug. Res. 2011, 3, 1–7. [Google Scholar]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Recent trends in the chemistry of 2-aminobenzothiazoles. J. Sulphur. Chem. 2009, 3, 74–107. [Google Scholar] [CrossRef]
- Priyanka; Sharma, N.K.; Keshari, K.J. Benzothiazole: The molecule of diverse biological activities. Int. J. Pharm. Sci. Drug. Res. 2010, 2, 2010. [Google Scholar]
- Kumbhare, R.M.; Dadmal, T.; Kosurkar, U.; Sridhar, V.; Rao, J.V. Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazole derivatives: A novel class of cytotoxic agents. Bio. Med. Chem.Lett. 2012, 22, 453–455. [Google Scholar] [CrossRef]
- Hutchinson, I.; Bradshaw, T.; Matthews, C.S.; Stevens, M.F.; Westwell, A.D. Antitumour benzothiazoles. Part 20: 3'-cyano and 3' alkynyl-substituted 2-(4'-aminophenyl)benzothiazoles as new potent and selective analogues. Bio. Med. Chem.Lett. 2003, 13, 471–474. [Google Scholar] [CrossRef]
- Prouilic, C.; Vicendo, P.; Garrigues, J.; Poteeau, R.; Rima, G. Evaluation of new thiadiazoles and benzothiazole as potential radioprotectors: Free radical scavenging activity in vitro and theoretical studies (QSAR, DFT). Free. Radic. Biol. Med. 2009, 46, 1139–1148. [Google Scholar] [CrossRef]
- Amtul, Z.; Atta-ur-rehman; Siddiqui, R.A.; Choudhary, M.I. Chemistry and Mechanism of Urease Inhibition. Curr. Med. Chem. 2002, 9, 1323–1348. [Google Scholar] [CrossRef]
- Mobley, H.L.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar]
- Miyaura, N.; Yanagi, N.; Suzuki, A. The palladium-catalyzed cross coupling reaction of phenyl boronic acid with haloarenes in the presence of bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lamaire, M.C. Aryl-Aryl bond formation one century after the discovery of Ullmann reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef]
- Martin, A.R.; Yung, Y. Palladium-catalyzed cross-couplingreactions of organoboronic acids with organic electrophiles. Acta Chem. Scand. 1993, 47, 221–230. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium catalysts for the Suzuki Cross-Coupling Reaction: An overview of recent advances. J. Synth. Org.Chem. 2004, 15, 2419–2440. [Google Scholar]
- Piscitelli, F.; Ballatore, C.; Smith, I.A. Solid phase synthesis of 2-aminobenzothiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 644–648. [Google Scholar] [CrossRef]
- Amtul, Z.; Rasheed, M.; Choudhary, M.; Rosanna, S.; Khan, K.; Atta-ur-Rehman. Kinetics of novel competitive inhibitors of urease enzymes by a focused library of oxadiazoles/thiadiazoles and triazoles. Biochem. Biophys. Res. Commun. 2004, 319, 1053–1063. [Google Scholar] [CrossRef]
- Hassan, F. Synthesis, Characterization, Anti-inflammatory, and Antioxidant Activity Of Some New Thiazole Derivatives. Int. J. Appl. Sci. Techn. 2012, 2, 180–187. [Google Scholar]
- Majo, V.J.; Prabhakaran, J.; Mann, J.J.; Kumar, J.S.D. An efficient palladium catalyzed synthesis of 2-arylbenzothiazoles. Tetrahedron Letters. 2003, 44, 8535–8537. [Google Scholar] [CrossRef]
- Jimonet, P.; Andiau, F.; Barreau, M.; Blanched, J.C.; Boioreau, A.; Bour, Y.; Coleno, M.A.; Doble, A.; Deodflinger, G.; Huu, D.C.; Donat, M.H. Synthesis in vivo “Antiglutamate” Activity of 6-Substituated- 2- benzothiazolamine and 3- Substituted-2-imino-benzothiazolines. J. Med. Chem. 1999, 42, 2828–2843. [Google Scholar] [CrossRef]
- Martin, M.T.; Roschanger, F.; Eddy, J.F. Practical acid Catalyzed acylation of sulfonaimide with cadboxylic acid anhydrides. Tetrahedron. Lett. 2003, 44, 5461–5483. [Google Scholar] [CrossRef]
- Serwar, M.; Akhtar, T.; Hameed, S.; Khan, K.M. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryle-4- (1- Phenylpropyl)-2H-1,2,4-Trizole- 3(4H)- thiones. ARKIVOC 2009, vii, 210–221. [Google Scholar]
- Pervez, H.; Rmzan, M.; Yaqub, M.; Nasim, F.H.; Khan, K.M. Synthesis and Biological Evaluation of Some New N4-Aryl Substituted 5-Chloroisatin-3-thiosemicarbazones. Med. Chem. 2012, 8, 505–514. [Google Scholar] [CrossRef]
- Munakata, K.; Tanaka, S.; Toyoshima, S. Therapy for urolithiasis with hydroxamic acids. I. Synthesis of new N-(aroyl)glycinohydroxamic acid derivatives and related compounds. Chem. Pharm. Bull. 1980, 28, 2045–2051. [Google Scholar] [CrossRef]
- Hase, J.; Kobashi, K. Inhibition of Proteus vulgaris urease by hydroxamic acids. J. Biochem. 1967, 62, 293–299. [Google Scholar]
- Fishbein, W.N.; Carbone, P.P. Urease Catalysis: II. Inhibition of the enzyme by Hydroxyurea, hydroxylamine, and acetohydroxamic acid. J. Biol Chem. 1965, 240, 2407–2409. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Vamanu, V. Studies of the Antioxidant and Antimicrobial activities of Pleurotus odstreatus psi101109 mycellium. Pak. J. Bot. 2013, 45, 311–317. [Google Scholar]
- Naresh, P.; Pattanaik, P.; Rajeshwar, B. Synthetic Characterization and Antioxidant Screening of Some Novel 6-Fluorobenzothiazole Substituted [1,2,4] Triazole Analogues. Int. J. Pharm. Sci. 2013, 3, 170–174. [Google Scholar]
- Hazra, K.; Nargund, L.V.; Rashmi, P.; Chandra, N.; Nandha, B. Synthesis and antioxidant activity of some novel Fluorobenzothiazolopyrazoline. Der Chemica Sinica 2011, 2, 149–157. [Google Scholar]
- Marcocci, L.; Packer, L.; Droy-Lefai, M.T.; Sekaki, A.; Albert, M. Antioxidant action of Ginko biloba extracts EGb 761. Meth. Enzymol. 1994, 234, 462–475. [Google Scholar] [CrossRef]
- Garrat, D. The Quantitative Analysis of Drugs, 3rd ed.; Chapman and Hall Ltd.: Tokyo, Japan, 1964; pp. 456–458. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium- catalyzed cross-coupling reactions of oganoboron compound. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gull, Y.; Rasool, N.; Noreen, M.; Nasim, F.-u.-H.; Yaqoob, A.; Kousar, S.; Rashid, U.; Bukhari, I.H.; Zubair, M.; Islam, M.S. Efficient Synthesis of 2-Amino-6-Arylbenzothiazoles via Pd(0) Suzuki Cross Coupling Reactions: Potent Urease Enzyme Inhibition and Nitric Oxide Scavenging Activities of the Products. Molecules 2013, 18, 8845-8857. https://doi.org/10.3390/molecules18088845
Gull Y, Rasool N, Noreen M, Nasim F-u-H, Yaqoob A, Kousar S, Rashid U, Bukhari IH, Zubair M, Islam MS. Efficient Synthesis of 2-Amino-6-Arylbenzothiazoles via Pd(0) Suzuki Cross Coupling Reactions: Potent Urease Enzyme Inhibition and Nitric Oxide Scavenging Activities of the Products. Molecules. 2013; 18(8):8845-8857. https://doi.org/10.3390/molecules18088845
Chicago/Turabian StyleGull, Yasmeen, Nasir Rasool, Mnaza Noreen, Faiz-ul-Hassan Nasim, Asma Yaqoob, Shazia Kousar, Umer Rashid, Iftikhar Hussain Bukhari, Muhammad Zubair, and Md. Saiful Islam. 2013. "Efficient Synthesis of 2-Amino-6-Arylbenzothiazoles via Pd(0) Suzuki Cross Coupling Reactions: Potent Urease Enzyme Inhibition and Nitric Oxide Scavenging Activities of the Products" Molecules 18, no. 8: 8845-8857. https://doi.org/10.3390/molecules18088845






