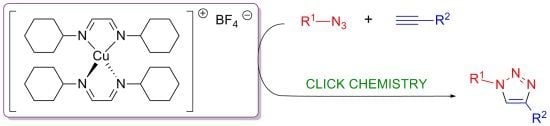
Well-Defined Diimine Copper(I) Complexes as Catalysts in Click Azide-Alkyne Cycloaddition Reactions
Abstract
:1. Introduction
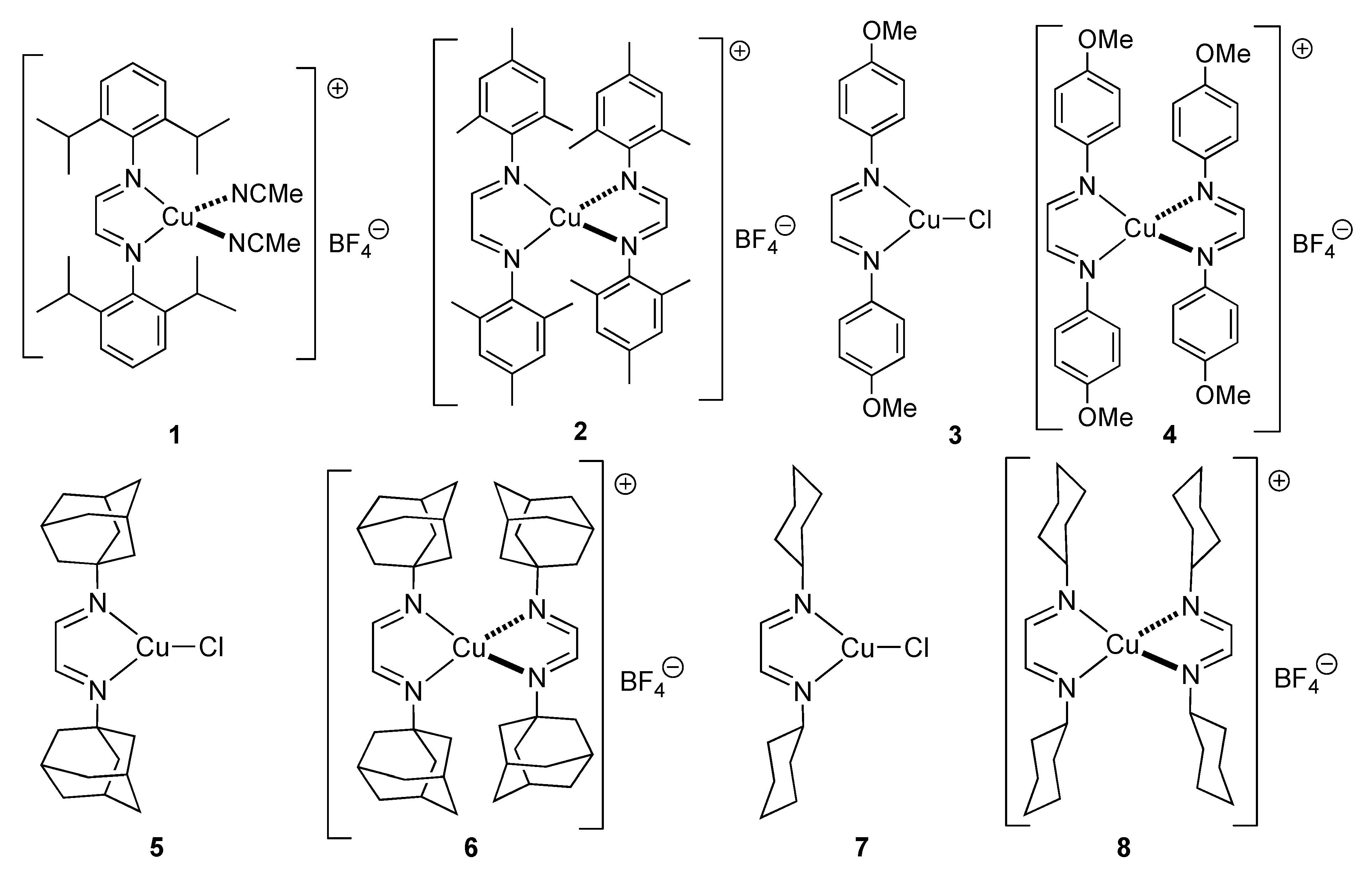
2. Results and Discussion
| Entry | Solvent | [Cu] (mol%) | Conv. (%) a |
|---|---|---|---|
| 1 | MeCN | 5 | <5 |
| 2 | Cyclohexane | 5 | <5 |
| 3 | Ethyl Acetate | 5 | <5 |
| 4 | MeOH | 5 | 52 |
| 5 | Neat | 5 | 13 |
| 6 | THF | 5 | 84 to <5 |
| 7 | Water/t-BuOH | 5 | >95 to 9 |
| 8 | Water | 5 | >95 |
| 9 | Acetone | 5 | >95 |
| 10 | Water | 2 | 52 |
| 11 | Acetone | 2 | 86 |

| Entry | [Cu] | Concentration (M) | Conv. (%) a |
|---|---|---|---|
| 1 | 5, 1 mol% | 0.5 | <95 |
| 2 | 7, 1 mol% | 0.5 | <95 |
| 3 | 8, 1 mol% | 0.5 | <95 |
| 4 | 5, 0.5 mol% | 0.5 | 63 |
| 5 | 7, 0.5 mol% | 0.5 | 92 |
| 6 | 8, 0.5 mol% | 0.5 | <95 |
| 7 | 7, 0.5 mol% | 1.0 | 93 |
| 8 | 8, 0.5 mol% | 1.0 | <95 |
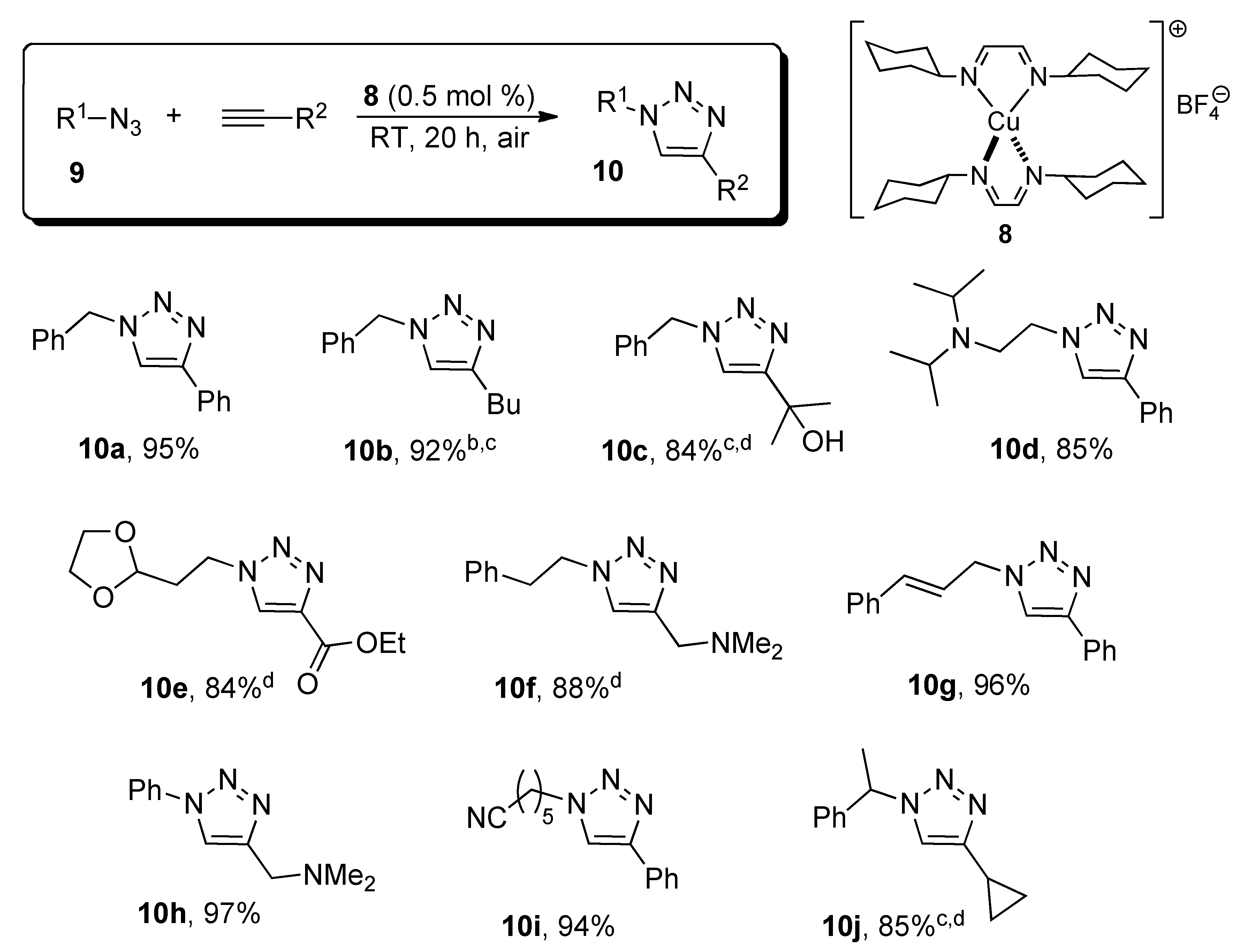
3. Experimental Section
3.1. General
3.2. Catalytic Results
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloaddition of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- L’abbe, G. Are azidocumulenes accessible? Bull. Soc. Chem. Belg. 1984, 93, 579–592. [Google Scholar] [CrossRef]
- Special Issue on Click Chemistry. Chem. Soc. Rev. 2010, 39, 1221–1408. [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Díez-González, S. Well-defined copper(I) complexes for Click azide-alkyne cycloaddition reactions: One Click beyond. Catal. Sci. Tech. 2011, 1, 166–178. [Google Scholar] [CrossRef]
- Donnelly, P.S.; Zanatta, S.D.; Zammit, S.C.; White, J.M.; Williams, S.J. “Click” cycloaddition catalysts: Copper(I) and copper(II) tris(triazolylmethyl)amine complexes. Chem. Commun. 2008, 2008, 2459–2461. [Google Scholar]
- Chan, T.R.; Fokin, V.V. Polymer-supported copper(i) catalysts for the experimentally simplified azide-alkyne cycloaddition. QSAR Comb. Sci. 2007, 26, 1274–1279. [Google Scholar] [CrossRef]
- Özçubukçu, S.; Ozkal, E.; Jimeno, C.; Pericàs, M.A. A highly active catalyst for Huisgen 1,3-dipolar cycloadditions based on the tris(triazolyl)methanol-Cu(I) structure. Org. Lett. 2009, 11, 4680–4683. [Google Scholar] [CrossRef]
- Candelon, N.; Lastécouères, D.; Diallo, A.K.; Aranzaes, J.R.; Astruc, D.; Vincent, J.M. A highly active and reusable copper(I)-tran catalyst for the “click” 1,3-dipolar cycloaddition of azides and alkynes. Chem. Commun. 2008, 2008, 741–743. [Google Scholar]
- Li, L.; Lopez, P.S.; Rosa, V.; Figueira, C.A.; Lemos, M.A.N.D.A.; Duarte, M.T.; Avilés, T.; Gomes, P.T. Synthesis and structural characterisation of (aryl-BIAN)copper(I) complexes and their application as catalysts for the cycloaddition of azides and alkynes. Dalton Trans. 2012, 41, 5144–5154. [Google Scholar] [CrossRef]
- Frutos Pedreño, R.; Markalain-Barta, J.; Vega Isa, E.; Díez-González, S. Manuscript in preparation. 2013. [Google Scholar]
- Alvarez, S.G.; Alvarez, M.T. A practical procedure for the synthesis of alkyl azides at ambient temperature in demethylsulfoxide in high purity and yield. Synthesis 1997, 1997, 413–414. [Google Scholar] [CrossRef]
- Engler, A.C.; Bonner, D.K.; Buss, H.G.; Cheung, E.Y.; Hammond, P.T. The synthetic tuning of clickable pH responsive cationic polypeptides and block copolypeptides. Soft Matter 2011, 7, 5627–5637. [Google Scholar] [CrossRef]
- Carboni, B.; Vaultier, M.; Carrié, R. Etude de la chimioselectivite de la reaction des dichloroboranes avec les azides fonctionnels: une synthese efficace d'amines secondaires fonctionnalisees. Tetrahedron 1987, 43, 1799–1810. [Google Scholar] [CrossRef]
- Van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; van Delft, F.L. Organophosphorus-catalysed staudinger reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
- Rehse, K.; Cwiklicki, A. Antiaggregating and antithrombotic activities of new 1,2,3-triazolecarboxamides. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 156–163. [Google Scholar] [CrossRef]
- Luo, L.; Wilhelm, C.; Sun, A.; Grey, C.P.; Lauher, J.W.; Goroff, N.S. Poly(diiododiacetylene): Preparation, isolation, and full characterization of a very simple poly(diacetylene). J. Am. Chem. Soc. 2008, 130, 7702–7709. [Google Scholar]
- Appukkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A microwave-assisted Click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(I)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef]
- Díez-González, S.; Nolan, S.P. [(NHC)2Cu]X complexes as efficient catalysts for azide-alkyne Click chemistry at low catalyst loadings. Angew. Chem. Int. Ed. 2008, 47, 8881–8884. [Google Scholar] [CrossRef]
- Kamijo, S.; Jin, T.; Huo, Z.; Yamamoto, Y. A one-pot procedure for the regioconrolled synthesis of allyltriazoles via the Pd-Cu bimetallic catalysed three-component coupling reaction of nonactivated terminal alkynes, allyl carbonate, and trimethylsilyl azide. J. Org. Chem. 2004, 69, 2386–2393. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhao, Y.B.; Fan, M.J.; Liu, W.M.; Liang, Y.M. General synthesis of (1-substituted-1H-1,2,3-triazol-4-ylmethyl)-dialkylamines via a copper(I)-catalyzed three-component reaction in water. Tetrahedron 2005, 61, 9331–9337. [Google Scholar] [CrossRef]
- Lal, S.; Díez-González, S. [CuBr(PPh3)3] for azide-alkyne cycloaddition reactions under strict Click conditions. J. Org. Chem. 2011, 76, 2367–2373. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barta, J.M.; Díez-González, S. Well-Defined Diimine Copper(I) Complexes as Catalysts in Click Azide-Alkyne Cycloaddition Reactions. Molecules 2013, 18, 8919-8928. https://doi.org/10.3390/molecules18088919
Barta JM, Díez-González S. Well-Defined Diimine Copper(I) Complexes as Catalysts in Click Azide-Alkyne Cycloaddition Reactions. Molecules. 2013; 18(8):8919-8928. https://doi.org/10.3390/molecules18088919
Chicago/Turabian StyleBarta, Jordi Markalain, and Silvia Díez-González. 2013. "Well-Defined Diimine Copper(I) Complexes as Catalysts in Click Azide-Alkyne Cycloaddition Reactions" Molecules 18, no. 8: 8919-8928. https://doi.org/10.3390/molecules18088919