In Vitro Anticariogenic Effects of Drymocallis rupestris Extracts and Their Quality Evaluation by HPLC-DAD-MS3 Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Polyphenolic Compounds
| Extracts | Total phenolic content (TPC) (mg GAE/g dry weight) a |
|---|---|
| PRU | 26.7 ± 0.8 |
| PRU1 | 23.2 ± 0.5 |
| PRU2 | 29.9 ± 0.7 |
| PRU3 | 30.9 ± 1.1 |
| PRU4 | 22.5 ± 0.9 |
| Polyphenols content (mg/g dry weight) a | ||||
|---|---|---|---|---|
| Phenolic acids (TPA) | Flavonoids (TFC) | Tannins (TTC) | Proanthocyanidins (TPDC) | |
| Quercetin | Hyperoside | |||
| 7.8 ± 0.4 | 4.2 ± 0.3 | 6.0 ± 0.5 | 115.0 ± 3.7 | 4.6 ± 0.4 |
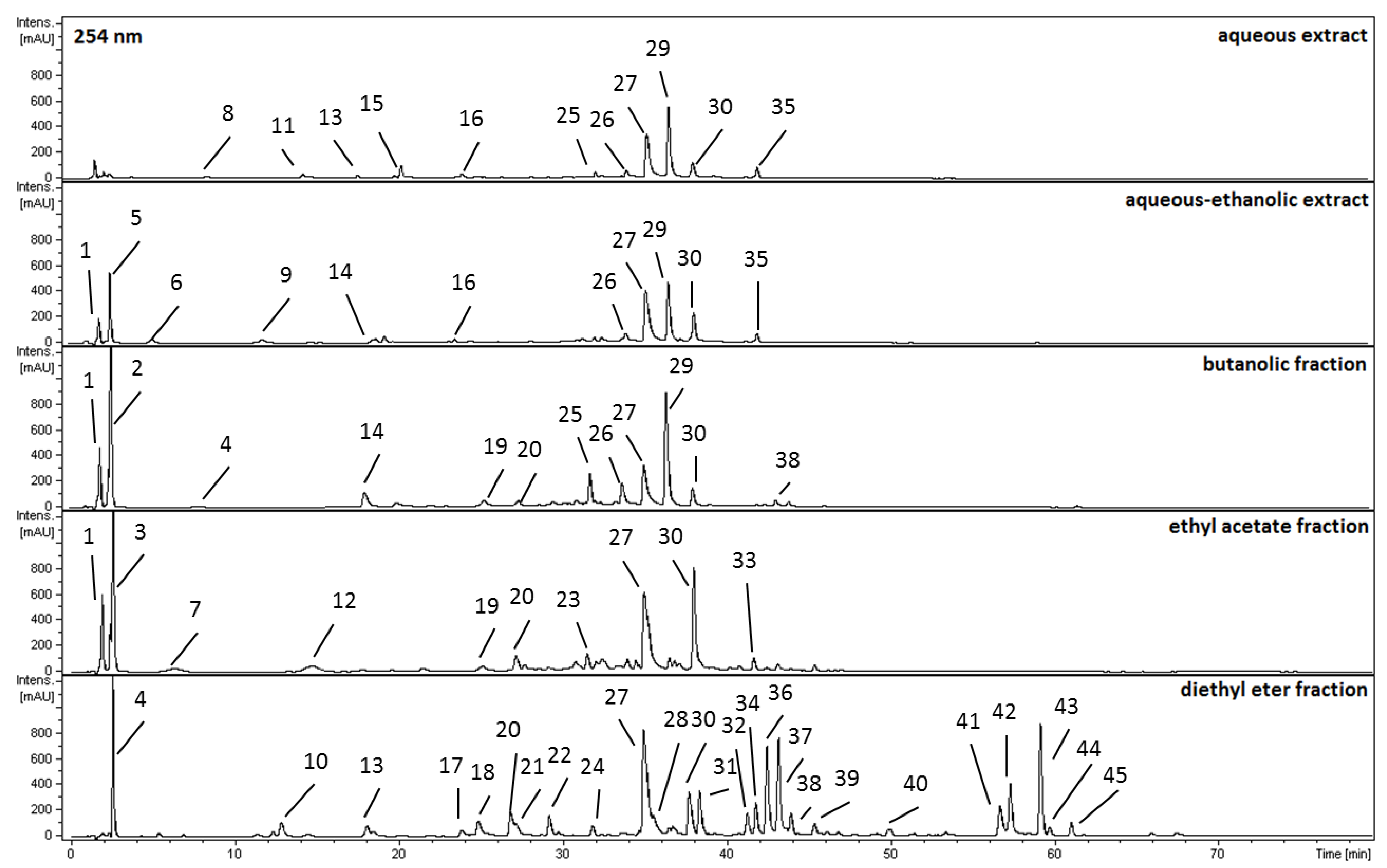
2.2. Anticariogenic activity
| Identified compounds | Retention time [min] | UV [nm] | [M-H]− m/z | MS2 ions | MS3 ions | [M+H]+ | MS2 ions | MS3 ions | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | HHDP-glucose | 2.1 | 260 | 481 | 421, 301b | - | - | - | - |
| 2 | unknown compound | 2.6 | 220, 258 | 639 | 613, 463b, 301 | - | 641 | - | - |
| 3 | unknown tannin | 2.7 | 257 | 783 | - | - | 785 | - | - |
| 4 | gallic acid * | 2.7 | 266 | 169 | 125b | - | 171 | 127b | - |
| 5 | unknown compound | 2.8 | 262 | 447 | 315b, 297, 207, 177, 163 | - | - | - | - |
| 6 | unknown tannin | 5.4 | 248 | 391a, 783 | 481, 421, 301b, 275 | - | 785 | 767b, 465, 303 | - |
| 7 | unknown tannin | 6.7 | 249 | 391a, 783 | 481, 421, 301b, 275 | - | 785 | 767b, 465, 303 | - |
| 8 | bis-HHDP-glucose isomer | 9.0 | 216, 260 sh | 783 | 481, 421, 301b | - | 785 | 767b, 465, 303 | - |
| 9 | bis-HHDP-glucose isomer | 12.2 | 250 | 783 | 481, 301b, 275 | - | - | - | - |
| 10 | methyl gallate * | 13.0 | 271 | 183 | 167b | - | 185 | 153b | - |
| 11 | bis-HHDP-glucose isomer | 14.8 | 216, 260 sh | 783 | 481, 421, 301b | - | 785 | 767b, 465, 303 | - |
| 12 | galloyl-HHDP-glucose isomer | 15.2 | 271 | 633 | 463, 301b | - | 657b | - | - |
| 13 | brevifolincarboxilic acid | 18.1 | 245, 296, 324 | 291 | 247b, 279 | - | 293 | 257, 169 | - |
| 14 | quercetin derivative | 18.3 | 260, 350 | 757 | 595b | 463, 445, 343, 301b | 759 | 627, 465b, 303 | 465b, 303 |
| 15 | quercetin glucuronylhexoside | 19.2 | 273, 353 | 639 | 463b, 301 | 343, 301b | 641 | 465b, 303 | 303b |
| 16 | caffeoylquinic acid isomer | 23.8 | 234 sh, 303 sh, 327 | 353 | 191, 173b, 155, 127, 111 | - | 377b | 243, 215b, 197, 185 | - |
| 17 | ellagic acid derivative | 24.0 | 255, 261 | 469 | 451, 425b, 301, 167 | - | 493b | 475b, 452, 409, 333 | - |
| 18 | unknown flavonoid | 25.1 | 259, 295 sh, 357 | 399 | 273b | - | 401 | 275b, 247 | - |
| 19 | ellagic acid 3,3'-di-O-methyl ether 4-O-xyloside * | 25.2 | 250, 325 | 461 | 415b, 301 | - | 463 | 445, 331b, 275 | 271, 257b, 169 |
| 20 | unknown compound | 27.3 | 274 | 247 | 219, 191b | - | 249 | 207b, 186 | - |
| 21 | unknown compound | 27.4 | 224 sh, 266, 302 | 275 | 257b, 229, 203 | - | 277 | 259b, 249, 215 | - |
| 22 | |||||||||
| 23 | unknown flavonoid | 31.6 | 270, 350 | 639 | 459b, 444, 315 | - | 641 | 479, 317b | 461, 413, 317b |
| 24 | unknown compound | 31.9 | 270, 360 | 365 | 183b, 153 | - | 367 | 335b, 303 | - |
| 25 | quercetin pentosylglucuronide | 32.5 | 258, 351 | 609 | 301b, 179 | - | 633b, 611 | 597, 479b, 303 | 303 |
| 26 | unknown flavonoid | 34.6 | 250, 351 | 447 | 369, 301b | - | 449 | 303b | 285b, 275, 258 |
| 27 | ellagic acid * | 35.5 | 253, 362 | 301 | 284, 257b, 185 | - | 303 | 285b, 248 | - |
| 28 | uknown compound | 35.9 | slope | 435 | 303b, 285, 177 | 285b, 177 | 459b | 441, 412, 356, 327b, 307, 251 | - |
| 29 | quercetin 3-O-glucuronide * | 37.0 | 252, 350 | 477 | 301b | 273, 179b, 151 | 479 | 303b | 285, 257b, 227, 165 |
| 30 | unknown compound | 38.1 | 226, 292 | 435 | 303b, 285, 177 | 285b, 177, 125 | - | - | - |
| 31 | unknown compound | 38.5 | 264, 290 sh, 350 | 457 | 273b, 245 | - | 459 | 427b, 275, 261 | - |
| 32 | quercetin 3-O-arabinoside * | 41.4 | 267, 351 | 433 | 301b | - | 435 | 303b | 285, 275, 257b, 207, 165 |
| 33 | unknown flavonoid pentoside | 41.7 | 250, 361 | 447 | 315b, 300 | 299 | 449 | 317b, 286 | 286 |
| 34 | kaempferol 3-O-glucoside * | 41.9 | 265, 340 | 447 | 327, 299, 285b, 255 | - | 449 | 287b | 269, 241, 213, 153, 121b |
| 35 | kaempferol 3-O-glucuronide * | 42.5 | 265, 348 | 461 | 285b | 267, 257b, 241, 229, 213, 197, 185 | 463 | 287b | 241, 213, 127b |
| 36 | ellagic acid derivative | 42.6 | 255, 366 | 483 | 451b, 407, 315, 301 | - | 485 | 471, 453b, 435 | - |
| 37 | valoneic acid dilactone methyl ester * | 43.3 | 255, 366 | 483 | 451b, 407, 315, 301 | - | 485 | 471, 453b, 435 | - |
| 38 | isorhamnetin glucuronide | 44.1 | 257, 265 sh, 355 | 491 | 473, 315b, 301 | 300b, 287, 272 | 493 | 303b | - |
| 39 | ellagic acid derivative | 45.3 | 257, 355 | 457 | 275b | - | 459 | 427, 409b, 399, 257 | - |
| 40 | unknown compound | 50.0 | 267, 315 | 445 | 265, 235, 205, 163, 145b | - | 469b | 377, 306, 173, 147b | - |
| 41 | quercetin* | 56.7 | 255, 368 | 301 | 273, 257, 229, 179b, 151 | - | 303 | 275b, 257, 230, 165 | - |
| 42 | unknown compound | 57.4 | 253, 267 | 483 | 251b, 301 | - | 485 | 453b | - |
| 43 | tiliroside* | 59.3 | 267, 311, 354 sh | 593 | 447, 285b | 285 | 595 | 329, 309, 287b, 165 | - |
| 44 | unknown flavonoid | 59.9 | 267, 315, 360 sh | 623 | 461, 447, 332b, 299, 285 | 341, 299, 285 | 625 | 339b, 321, 287, 177 | - |
| 45 | tiliroside isomer | 61.1 | 267, 308, 351 sh | 593 | 447, 307, 285b | 327, 285b, 255 | 595 | 392, 309, 287b | - |
| Tested strains | MIC (mg/mL) | MBC (mg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRU | PRU1 | PRU2 | PRU3 | PRU4 | CHX a | PRU | PRU1 | PRU2 | PRU3 | PRU4 | CHX a | |
| S. sobrinus/downei 21020 | >12 | >12 | 1.5 | >12 | >12 | 3.125 × 10−3 | >12 | >12 | 1.5 | >12 | >12 | 6.25 × 10−3 |
| S. sobrinus DSM 20381 | >12 | >12 | 0.75 | >12 | >12 | 3.125×10−3 | >12 | >12 | 1.5 | >12 | >12 | 3.125×10−3 |
| S. mutans 6067 | 6 | 6 | 1.5 | 6 | >12 | 3.125 × 10−3 | 12 | 12 | 3 | 12 | >12 | 3.125×10−3 |
| S. sobrinus 6070 | >12 | 6 | 1.5 | 6 | >12 | 3.125 × 10−3 | >12 | 12 | 3 | 12 | >12 | 6.25×10−3 |
| S. sanguis ATCC 10556 | >12 | 3 | 1.5 | 3 | >12 | 3.125 × 10−3 | >12 | 6 | 3 | 6 | >12 | 6.25×10−3 |
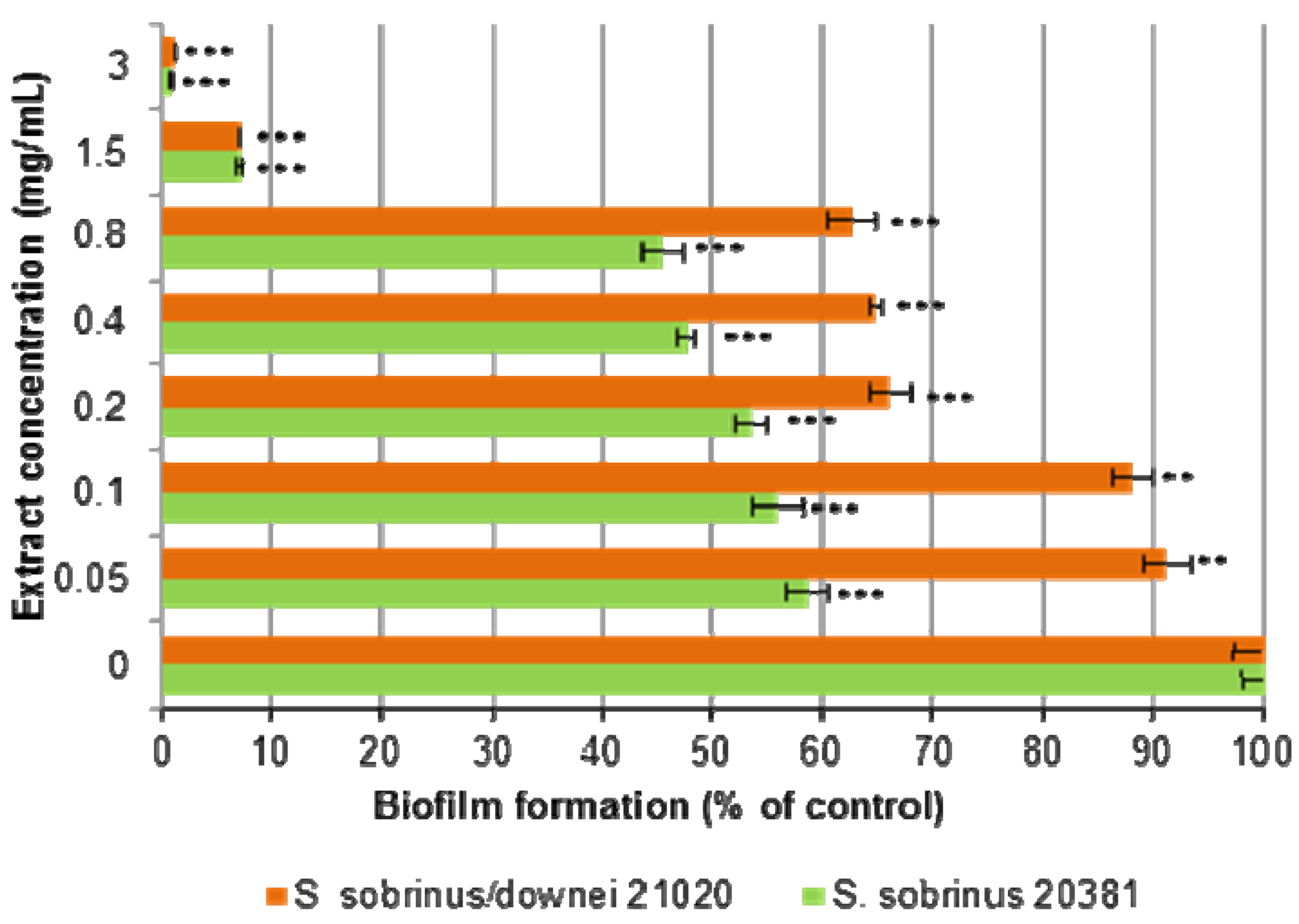
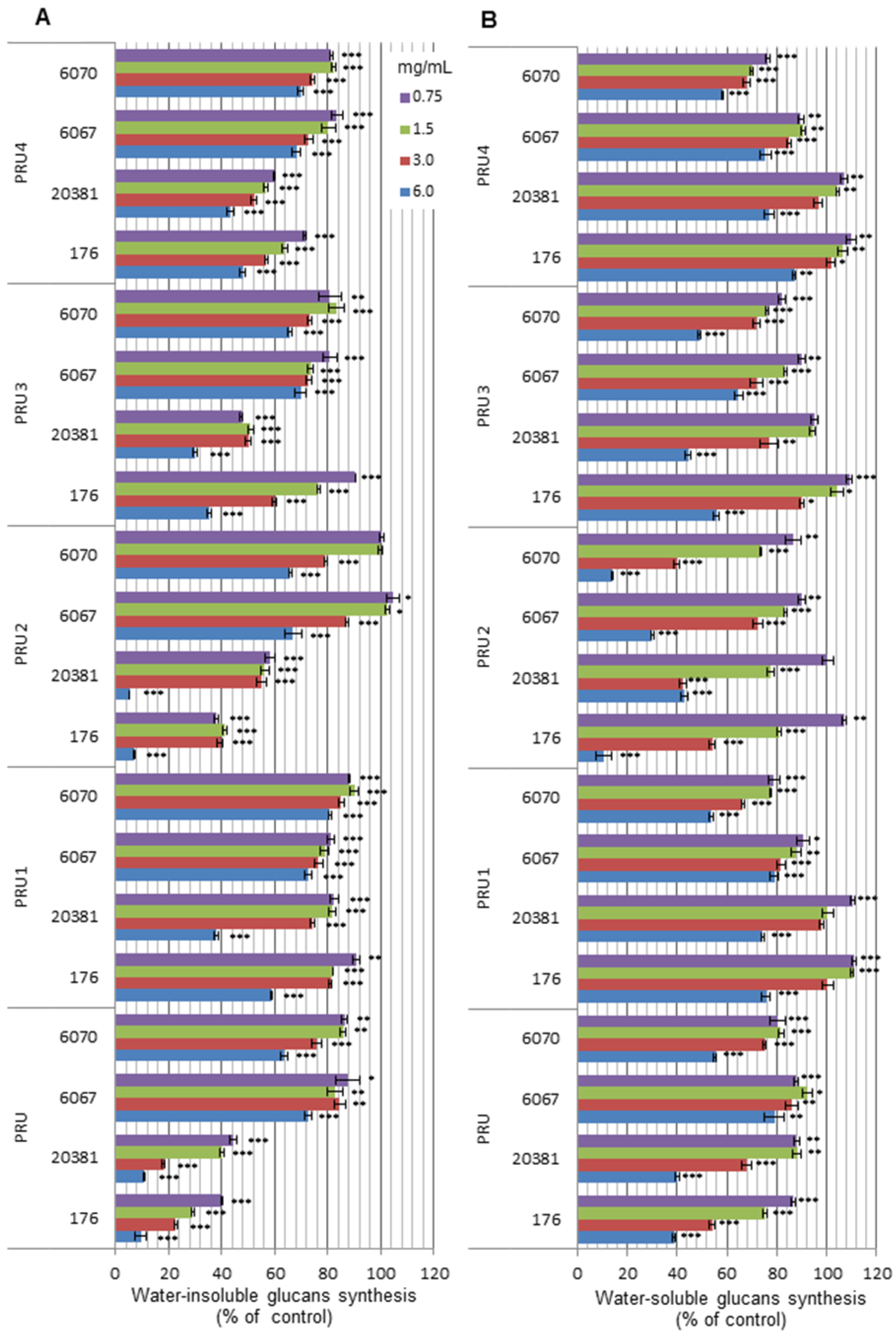
3. Experimental
3.1. Plant material
3.2. Preparation of Extracts and Their Subfractions
3.3. Phytochemical Profile
3.3.1. Determination of Total Polyphenol Content
3.3.2. Determination of Total Phenolic Acids Content
3.3.3. Determination of Total Flavonoid Content
3.3.4. Determination of Total Tannin Content
3.3.5. Determination of Total Proanthocyanidin Content
3.3.6. HPLC-DAD-MS3 Analysis
3.4. Anticariogenic Activity
3.4.1. Bacterial Strains
3.4.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.4.3. Inhibition of Biofilm Formation
3.4.4. Inhibition of streptococcal α-d-glucans synthesis
3.5. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kurtto, A.; Eriksson, T. Atlas Florae Europaeae notes. 15. Generic delimitation and nomenclatural adjustments in Potentilleae (Rosaceae). Ann. Bot. Fennici 2003, 40, 135–141. [Google Scholar]
- Wilson, G.B.; Whittington, W.J.; Humphries, R.N. Potentilla rupestris L. (Potentilla corsica Sieber ex Lehm). J. Ecology 1995, 83, 335–343. [Google Scholar] [CrossRef]
- Wilkes, S.; Glasl, H. Isolation, characterization, and systematic significance of 2-pyrone-4,6-dicarboxylic acid in Rosaceae. Phytochemistry 2001, 58, 441–449. [Google Scholar] [CrossRef]
- wieżewska, E.; Chojnacki, T. The occurrence of unique, long-chain polyprenols in the leaves of Potentilla species. Acta Biochim. Polon. 1989, 36, 143–158. [Google Scholar]
- Tomczyk, M. Secondary metabolites from Potentilla recta L. and Drymocallis rupestris (L.) Soják (syn. Potentilla rupestris L.) (Rosaceae). Biochem. Syst. Ecol. 2011, 39, 893–896. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla - A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef]
- Naylor, M.N. Diet and the prevention of dental caries. JR Soc. Med. 1986, 79, 11–14. [Google Scholar]
- Johnston, D.W. Current status of professionally applied topical fluorides. Community Dent. Oral Epidemiol. 1994, 22, 159–163. [Google Scholar] [CrossRef]
- Emilson, C.G. Potential efficacy of chlorhexidine against mutans steptococci and human dental caries. J. Dent. Res. 1994, 73, 682–691. [Google Scholar]
- Simonsen, R.J. Pit and fissure sealant: review of the literature. Pediatr. Dent. 2002, 24, 393–414. [Google Scholar]
- Childers, N.K.; Tong, G.; Li, F.; Dasanayake, A.P.; Kirk, K.; Michalek, S.M. Hummans immunized with Streptococcus mutans antigens by mucosal routes. J. Res. Dent. 2002, 81, 48–52. [Google Scholar] [CrossRef]
- Hillman, J.D.; Brooks, T.A.; Michalek, S.M. Construction and characterization of effector strain of Streptococcus mutans for replacement therapy of dental caries. J. Res. Dent. 2002, 68, 543–549. [Google Scholar]
- Palombo, E.A. Traditional medicine plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Alternat. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Tomczyk, M.; Pleszczyńska, M.; Wiater, A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticariogenic activity. Molecules 2010, 15, 4639–4651. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar]
- Bowen, W.H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia IX; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Christ, B.; Müller, K.H. Determination of the amount of flavonol derivatives in drugs. Archiv der Pharmazie 1960, 293, 1033–1042. [Google Scholar] [CrossRef]
- DAB 10, Deutsches Arzeneibuch, Amtliche Ausgabe; Deutcher Apotheker Verlag: Stuttgart, Germany, 1998; p. 197.
- European Pharmacopoeia (Ph. Eur.), 6th ed.; Council of Europe: Strasbourg, France, 2007.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–353. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts and subfractions are available from the authors (M.T.).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tomczyk, M.; Pleszczyńska, M.; Wiater, A.; Granica, S. In Vitro Anticariogenic Effects of Drymocallis rupestris Extracts and Their Quality Evaluation by HPLC-DAD-MS3 Analysis. Molecules 2013, 18, 9117-9131. https://doi.org/10.3390/molecules18089117
Tomczyk M, Pleszczyńska M, Wiater A, Granica S. In Vitro Anticariogenic Effects of Drymocallis rupestris Extracts and Their Quality Evaluation by HPLC-DAD-MS3 Analysis. Molecules. 2013; 18(8):9117-9131. https://doi.org/10.3390/molecules18089117
Chicago/Turabian StyleTomczyk, Michał, Małgorzata Pleszczyńska, Adrian Wiater, and Sebastian Granica. 2013. "In Vitro Anticariogenic Effects of Drymocallis rupestris Extracts and Their Quality Evaluation by HPLC-DAD-MS3 Analysis" Molecules 18, no. 8: 9117-9131. https://doi.org/10.3390/molecules18089117






