In Vitro Antiprotozoal Activity of Triterpenoid Constituents of Kleinia odora Growing in Saudi Arabia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemistry
2.2. Antiprotozoal Activity
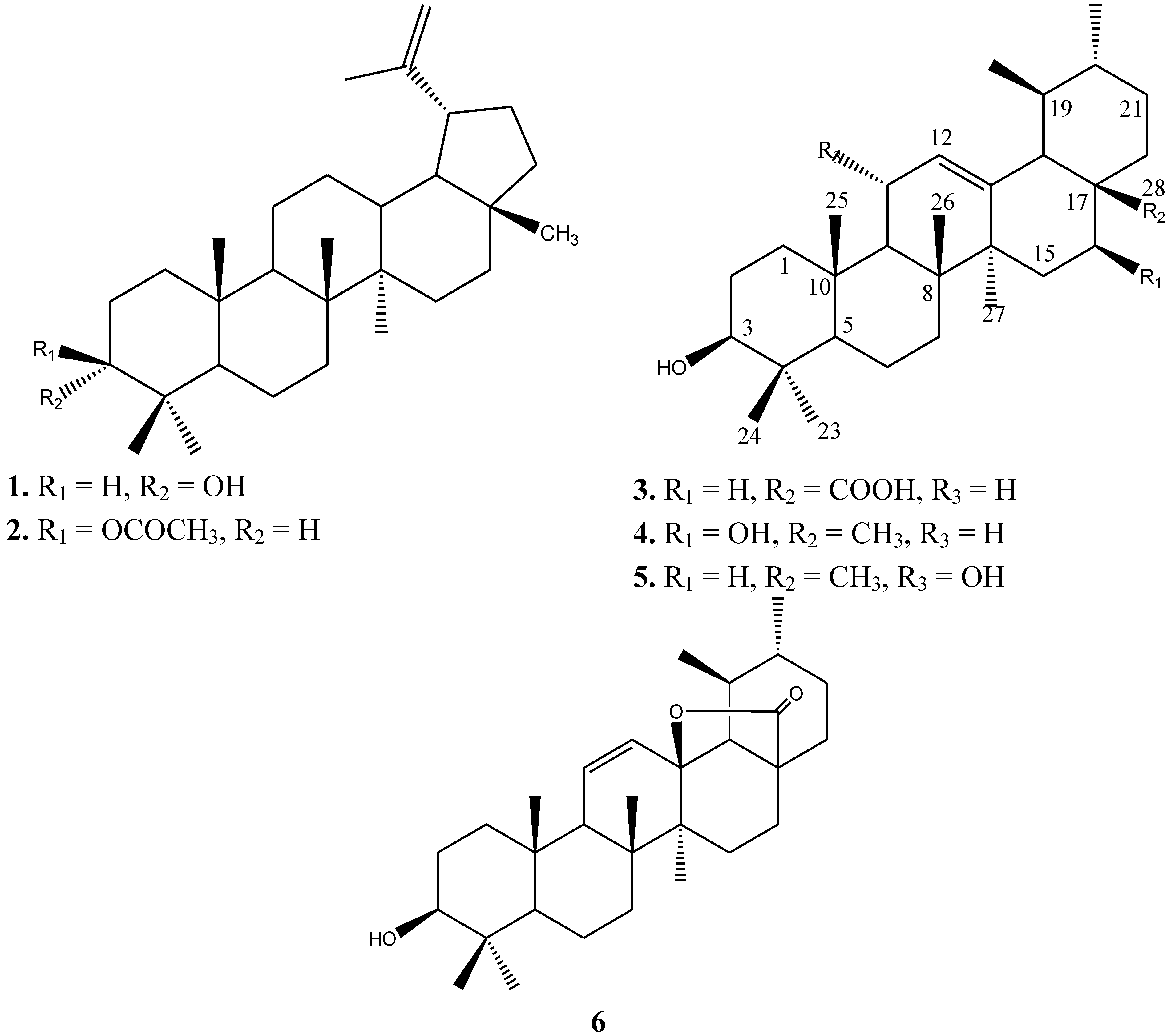
| # | Compound 1 in CDCl3 | Compound 2 in CDCl3 | Compound 3 in DMSO | Compound 4 in MeOD | Compound 5 in CDCl3 | Compound 6 in MeOD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | |
| 1 | 33.2 | 38.4 | 38.2 | 40.1 | 39.7 | 39.4 | ||||||
| 2 | 25.1 | 21.3 | 28.2 | 27.9 | 27.4 | 27.7 | ||||||
| 3 | 76.3 | 3.39 (br s) | 81.0 | 4.47 (dd, 5.5, 10.5 Hz) | 76.8 | 79.7 | 3.18 (d, 7.5 Hz) | 78.8 | 3.23 (br s) | 79.5 | 3.2 (dd, 6.5, 10 Hz) | |
| 4 | 37.5 | 38.0 | 38.8 | 39.9 | 39.1 | 40.0 | ||||||
| 5 | 49.0 | 55.4 | 54.8 | 56.7 | 55.3 | 56.1 | ||||||
| 6 | 18.3 | 18.2 | 18.0 | 19.5 | 18.4 | 18.8 | ||||||
| 7 | 34.1 | 34.2 | 32.7 | 31.8 | 33.5 | 32.3 | ||||||
| 8 | 41.0 | 40.8 | 40.0 | 41.0 | 43.3 | 41.5 | ||||||
| 9 | 50.1 | 50.3 | 47.1 | 48.5 | 55.8 | 2.2 (d, 10 Hz) | 54.0 | |||||
| 10 | 37.2 | 37.8 | 36.7 | 36.8 | 38.1 | 37.5 | ||||||
| 11 | 20.8 | 20.9 | 22.9 | 24.2 | 68.3 | 4.26 | 129.9 | 5.61 (d, 8.5 Hz) | ||||
| 12 | 25.3 | 23.7 | 124.5 | 5.16 (br s) | 126.3 | 5.23 (br s) | 128.6 | 5.18 (d, 2 Hz) | 135.0 | 6.07 (d, 10 Hz) | ||
| 13 | 38.0 | 37.1 | 138.2 | 139.7 | 143.0 | 91.9 | ||||||
| 14 | 42.9 | 42.8 | 41.6 | 45.1 | 42.1 | 42.0 | ||||||
| 15 | 27.4 | 25.1 | 27.2 | 36.4 | 27.9 | 26.6 | ||||||
| 16 | 35.6 | 35.6 | 25.6 | 67.4 | 4.19 (d, 6.5 Hz) | 27.4 | 23.9 | |||||
| 17 | 43.0 | 43.0 | 46.7 | 39.5 | 33.6 | 46.6 | ||||||
| 18 | 48.3 | 48.3 | 52.4 | 2.11 (d, 11.5 Hz) | 62.4 | 58.0 | 61.8 | |||||
| 19 | 48.0 | 2.38 (td, 11, 10, 5.5 Hz) | 48.0 | 2.38 (td, 11, 10, 5.5 Hz) | 38.4 | 40.9 | 39.3 | 41.5 | ||||
| 20 | 150.9 | 151.0 | 38.1 | 40.0 | 39.4 | 39.4 | ||||||
| 21 | 29.8 | 29.8 | 30.4 | 31.8 | 31.1 | 32.3 | ||||||
| 22 | 40.0 | 40.0 | 36.5 | 36.8 | 41.3 | 32.4 | ||||||
| 23 | 28.3 | 0.82 (s) | 27.4 | 0.85 (s) | 21.1 | 0.90 (s) | 28.8 | 0.82 (s) | 28.7 | 28.3 | 0.96 (s) | |
| 24 | 22.2 | 0.93 (s) | 16.5 | 0.84 (s) | 16.9 | 0.68 (s) | 16.3 | 1.02 (s) | 23.1 | 1.29 (s) | 15.6 | 0.80 (s) |
| 25 | 16.0 | 0.84 (s) | 16.2 | 1.05 (s) | 15.1 | 0.87 (s) | 16.4 | 1.02 (s) | 16.8 | 0.90 (s) | 19.4 | 1.05 (s) |
| 26 | 15.9 | 1.03 (s) | 16.0 | 0.83 (s) | 16.9 | 0.76 (s) | 18.3 | 1.10 (s) | 18.0 | 1.26 (s) | 18.4 | 0.98 (s) |
| 27 | 14.6 | 0.96 (s) | 14.5 | 0.79 (s) | 23.3 | 1.10 (s) | 25.1 | 1.20 (s) | 23.1 | 1.30 (s) | 16.6 | 1.25 (s) |
| 28 | 18.0 | 0.78 (s) | 18.0 | 0.94 (s) | 178.6 | 22.1 | 0.80 (s) | 28.2 | 1.10 (s) | 182.6 | - | |
| 29 | 109.3 | 4.69 (br s), 4.57 (br s) | 109.3 | 4.69 (br s), 4.57 (br s) | 16.9 | 0.82, (d, 6.5 Hz) | 17.0 | 0.84 (d, 6.5 Hz) | 17.6 | 0.90 (d) | 18.3 | 1.05 (d, 7.5 Hz) |
| 30 | 19.3 | 1.64 | 19.3 | 1.68 (s) | 21.1 | 0.92, (d, 7.0 Hz) | 21.1 | 0.97 (d) | 21.4 | 0.95 (d) | 19.6 | 0.98 (d, 6.0 Hz) |
| 1’ | 171.0 | |||||||||||
| 2’ | 27.9 | 2.04 (s) | ||||||||||
| Sample | P. falciparum | L. infantum | T. cruzi | T. brucei | MRC-5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | |
| Petroleum ether extract | 8.6 ± 2.1 | 2.3 | 6.8 ± 1.6 | 3 | 5.7 ± 1.6 | 3.4 | 0.5 ± 0.1 | 39 | 19.4 ± 3.4 |
| Chloroform extract | 8.2 ± 1.9 | 4 | 8.1 ± 2.3 | 4 | 31.0 ± 4.9 | - | 0.5 ± 0.1 | 63 | 31.3 ± 4.2 |
| Cmpd. 3 | 29.7 ± 5.9 | <1 | 7.4 ±1.9 | 1.5 | 8.8 ± 2.3 | 1.3 | 2.2 ± 0.6 | 5.2 | 11.4 ± 2.1 |
| Cmpd. 4 | 9.7 ± 3.2 | >6.6 | 9.3 ± 2.2 | >6.9 | 9.9 ± 2.6 | >6.5 | 2.3 ± 0.4 | >27.8 | >64.0 |
| Cmpd. 5 | 23.9 ± 5.7 | 2.7 | 3.2 ± 0.9 | >20 | 8.1 ± 1.8 | >7.9 | 7.8 ± 1.8 | >8.2 | >64.0 |
| Cmpd. 6 | >64.0 | - | >64.0 | - | >64.0 | - | 40.9 ± 8.1 | - | >64.0 |
| Chloroquine | 0.3 ± 0.05 | ||||||||
| Miltefosine | 10.4 ± 2.1 | ||||||||
| Benznidazole | 1.9 ± 0.3 | ||||||||
| Suramine | 0.03 ± 0.01 | ||||||||
| Tamoxifen | 11.4 ± 3.2 | ||||||||
3. Experimental
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Antiprotozoal Assay
3.4.1. Standard Drugs
3.4.2. Biological Assays
3.4.3. Antiplasmodial Activity
3.4.4. Antileishmanial Activity
3.4.5. Antitrypanosomal Activity
3.4.6. Cytotoxicity against MRC-5 Cells
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kondrashin, A.V.; Baranova, A.M.; Morozova, L.F.; Stepanova, E.V. Urgent tasks of malaria elimination programs. Med. Parazitol. (Mosk) 2011, 3, 3–9. [Google Scholar]
- Tengku, S.A.; Norhayati, M. Public health and clinical importance of amoebiasis in Malaysia: A review. Trop. Biomed. 2011, 28, 194–222. [Google Scholar]
- Kondrashin, A.V.; Baranova, A.M.; Morozova, L.F.; Stepanova, E.V. Global trends in malaria control. Progress and topical tasks in malaria control programs. Med. Parazitol. (Mosk) 2011, 4, 3–8. [Google Scholar]
- Ferrari, B.C.; Cheung-Kwok-Sang, C.; Beggs, P.J.; Stephens, N.; Power, M.L.; Waldron, L.S. Molecular epidemiology and spatial distribution of a waterborne cryptosporidiosis outbreak in Australia. Appl. Environ. Microbiol. 2011, 77, 7766–7771. [Google Scholar] [CrossRef]
- Nwaka, S.; Ridley, R.G. Virtual drug discovery and development for neglected diseases through public private partnerships. Nat. Rev. Drug Discov. 2003, 2, 919–928. [Google Scholar] [CrossRef]
- Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdalla, W.E.; Schmidt, T.J. The antiprotozoal activity of methylated flavonoids from Ageratum conyzoides L. J. Ethnopharmacol. 2010, 129, 127–130. [Google Scholar] [CrossRef]
- Halliday, P. Kleinia saginata Compositae. Curtis's Bot. Mag. 1989, 6, 151–156. [Google Scholar] [CrossRef]
- Hulin, M. New species and combinations in Kleinia (Asteraceae) from the Horn of Africa. Nord. J. Bot. 2002, 22, 419–426. [Google Scholar]
- Halliday, P. The genus Klenia (Compositae) in Arabia. Kew Bull. 1983, 39, 817–827. [Google Scholar] [CrossRef]
- Collenette, S. An Illustrated Guide to the Flowers of Saudi Arabia; Scorpion Publishing Ltd.: London, UK, 1985. [Google Scholar]
- Migahid, A.M. Flora of Saudi Arabia, 3rd ed.; King Saud University Press: Riyadh, Saudi Arabia, 1996; Volume 2, p. 256. [Google Scholar]
- Bohlmann, F.; Knoll, K.-H.K. Zwei neue acylpyrrole aus Kleinia kleinioides. Phytochemistry 1978, 17, 599–601. [Google Scholar] [CrossRef]
- Bohlmann, F.; Suding, H. Weitere abrotanifolon-derivate aus Kleinia tomentosa. Phytochemistry 1980, 19, 687–688. [Google Scholar] [CrossRef]
- Bohlmann, F.; Ahmed, M.; Jakupovic, J.; Jeffrey, C. Sesquiterpenes from Kleinia species. Phytochemistry 1981, 20, 201–251. [Google Scholar]
- Dominguez-Carmona, D.B.; Escalante-Erosa, F.; Garcia-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef]
- Fukuda, Y.; Yamada, T.; Wada, S.I.; Sakai, K.; Matsunaga, S.; Tanaka, R. Lupane and oleanane triterpenoids from the cones of Liquidamber styraciflua. J. Nat. Prod. 2006, 69, 142–144. [Google Scholar] [CrossRef]
- Thu, V.K.; Kiem, P.V.; Minh, C.V.; Yen, P.H.; Cuong, N.X.; Huong, H.T. A new flavan glucoside from Glochidion eriocarpum. J. Chem. 2010, 48, 125–131. [Google Scholar]
- Waterman, P.G.; Ampofo, S. Dammarane triterpenes from the stem bark of Commiphora dazielii. Phytochemistry 1985, 24, 2925–2928. [Google Scholar] [CrossRef]
- Jamal, A.K.; Yaacob, W.A.; Din, L.B. A Chemical Study on Phyllanthus reticulates. J. Phys. Sci. 2008, 19, 45–50. [Google Scholar]
- Jang, D.S.; Kim, J.M.; Lee, G.Y.; Kim, J.H.; Kim, J.S. Ursane-type triterpenoids from the Aerial parts of Potentilla discolour. Agric. Chem. Biotechnol. 2006, 49, 48–50. [Google Scholar]
- Lima, M. da P.; Braga, P.A.; Macedo, M. L.; Silva, M. F.; Ferreira, G.; Fernandes, J.B.; Vieira, P.C. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia and Dacryodes hopkinsii: Chemosystematic Implications. J. Braz. Chem. Soc. 2004, 15, 385–394. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. A hydroxygermacrene and other constituents from Pseudobrickellia brasiliensis. Phytochemistry 1984, 23, 1798–1799. [Google Scholar] [CrossRef]
- Wang, H.; Fujimoto, Y. Triterpene esters from Eucalyptus tereticornis. Phytochemistry 1993, 33, 151–153. [Google Scholar]
- Bero, J.; Hannaert, V.; Chataigné, G.; Hérent, M.F.; Quetin-Leclercq, J. In vitro antitrypanosomal and antileishmanial activity of plants used in Benin in traditional medicine and bio-guided fractionation of the most active extract. J. Ethnopharmacol. 2011, 137, 998–1002. [Google Scholar] [CrossRef]
- Hoet, S.; Pieters, L.; Muccioli, G.G.; Habib-Jiwan, J.L.; Opperdoes, F.R.; Quetin-Leclercq, J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J. Nat. Prod. 2007, 70, 1360–1363. [Google Scholar] [CrossRef]
- Cunha, W.R.; Martins, C.; Ferreira, D.D.; Crotti, A.F.M.; Lopes, N.P.; Albuquerque, S. In vitro trypanocidal activity of Triterpenes from Miconia species. Planta Med. 2003, 69, 470–472. [Google Scholar] [CrossRef]
- Moulisha, B.; Kumar, G.A.; Kanti, H.P. Anti-leishmanial and anti-cancer activities of a pentacyclic triterpenoid isolated from the leaves of Terminalia arjuna Combretaceae. Trop. J. Pharm. Res. 2010, 9, 135–140. [Google Scholar]
- Cimanga, R.K.; Tona, G.L.; Mesia, G.K.; Kambu, O.K.; Bakana, D.P.; Kalenda, P.D.T.; Penge, A.O.; Muyembe, J.J.T.; Totte, J.; Pieters, L.; et al. Bioassayguided isolation of antimalarial triterpenoid acids from the leaves of Morinda lucida. Pharmaceut. Biol. 2006, 44, 677–681. [Google Scholar] [CrossRef]
- van Baren, C.; Anao, I.; Lira, P.D.; Debenedetti, S.; Houghton, P.; Croft, S.; Martino, V. Triterpenic acids and flavonoids from Satureja parvifolia. Evaluation of their antiprotozoal activity. Z. Naturforsch C. 2006, 61, 189–192. [Google Scholar]
- Peixoto, J.A; Silva, M.L.A.; Crotti, A.E.M.; Veneziani, R.C.S.; Gimenez, V.M.M.; Januário, A.H.; Groppo, M.; Magalhães, L.G.; Santos, F.F.; Albuquerque, S.; et al. Antileishmanial activity of the hydroalcoholic extract of Miconia langsdorffii, Isolated compounds, and semi-synthetic Derivatives. Molecules 2011, 16, 1825–1833. [Google Scholar] [CrossRef]
- Sidana, J.; Singh, S.; Arora, S.K.; Foley, W.J.; Singh, I.P. Terpenoidal constituents of Eucalyptus loxophleba ssp. Lissophloia. Pharmaceut. Biol. 2012, 50, 823–827. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro proof-of-concept. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef]
- Raz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue asssay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense, T. b. gambiense) in vitro. Acta Trop 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; la Flamme, A.C.; van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. (Bethesda) 1996, 40, 2592–2597. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al Musayeib, N.M.; Mothana, R.A.; Gamal, A.A.E.; Al-Massarani, S.M.; Maes, L. In Vitro Antiprotozoal Activity of Triterpenoid Constituents of Kleinia odora Growing in Saudi Arabia. Molecules 2013, 18, 9207-9218. https://doi.org/10.3390/molecules18089207
Al Musayeib NM, Mothana RA, Gamal AAE, Al-Massarani SM, Maes L. In Vitro Antiprotozoal Activity of Triterpenoid Constituents of Kleinia odora Growing in Saudi Arabia. Molecules. 2013; 18(8):9207-9218. https://doi.org/10.3390/molecules18089207
Chicago/Turabian StyleAl Musayeib, Nawal M., Ramzi A. Mothana, Ali A. El Gamal, Shaza M. Al-Massarani, and Louis Maes. 2013. "In Vitro Antiprotozoal Activity of Triterpenoid Constituents of Kleinia odora Growing in Saudi Arabia" Molecules 18, no. 8: 9207-9218. https://doi.org/10.3390/molecules18089207




