Anti-Obesity Effect of Artemisia capillaris Extracts in High-Fat Diet-Induced Obese Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. TPC and Antioxidant Activity in Different Extracts of A. capillaris
| Ethanol Concentration (%, v/v) | Yield (%) | TPC (mg GAE/g) | RSC (%) | Scoparone (mg/g) |
|---|---|---|---|---|
| 100 | 10.4 | 86.8 ± 0.09 | 61.9 ± 1.00 | 1.16 ± 0.13 |
| 70 | 15.2 | 99.8 ± 0.03 | 63.4 ± 1.49 | 1.97 ± 0.09 |
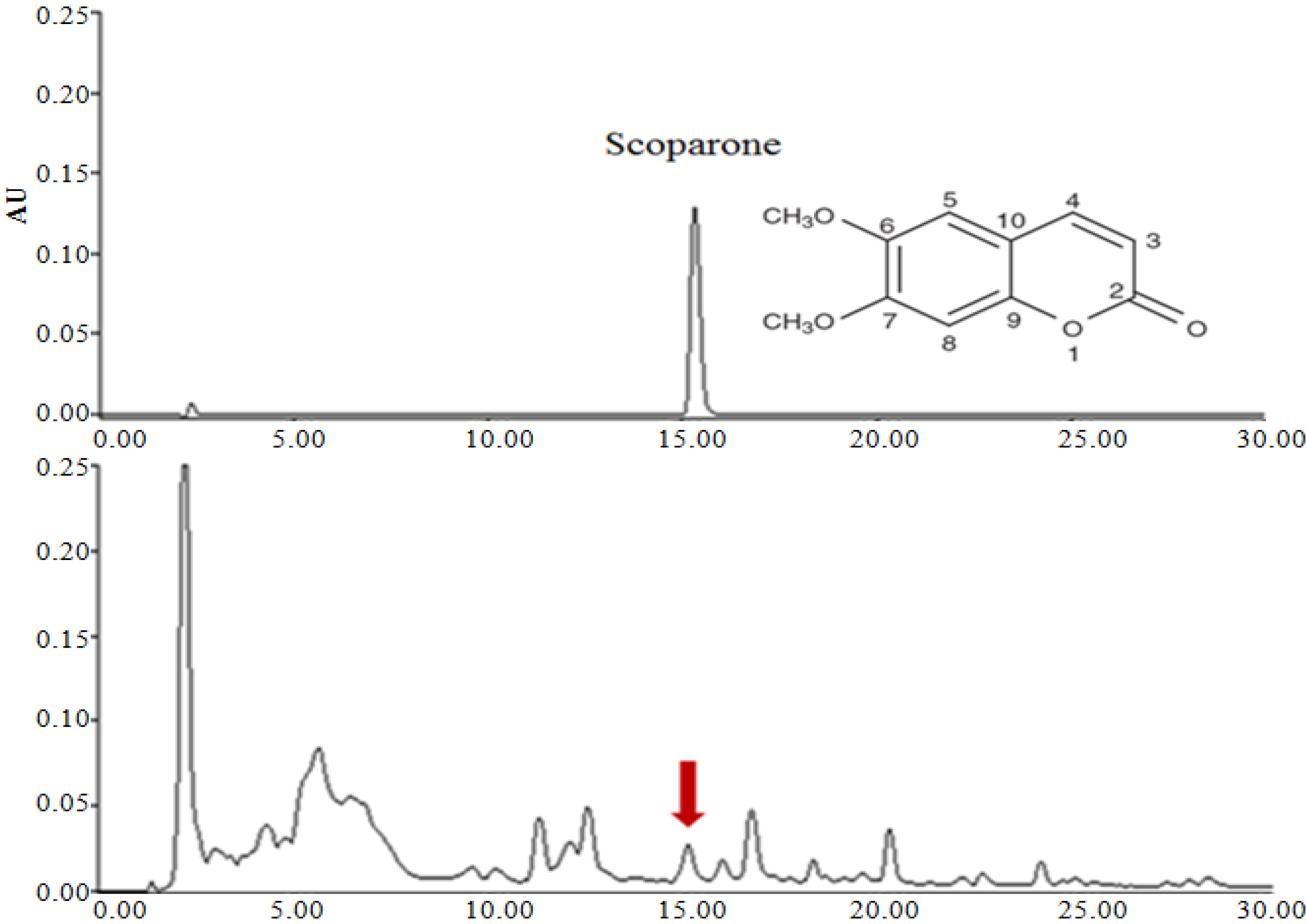
| 50 | 15.8 | 139.2 ± 0.40 a | 74.1 ± 0.75 a | 2.81 ± 0.21 a |
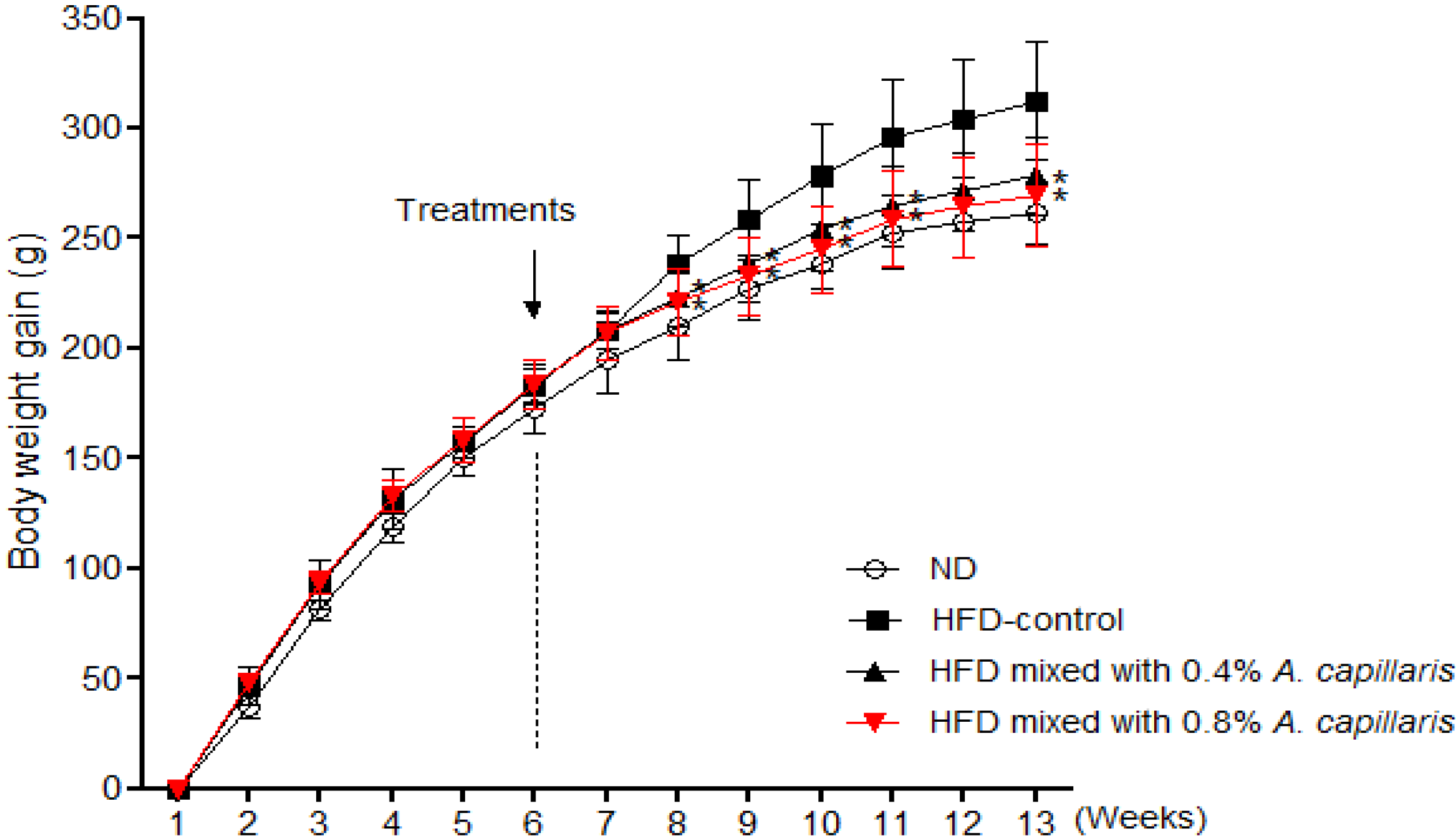
2.2. Effects of A. capillaris Extracts on Body Weight Gain and Food Intake
| Groups | Body weight (g) | Body weight gain (g/day) | Food intake (g/day) | Energy intake (kcal/day) | Epididymal adipose tissue (g) | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| I | 181.4 ± 8.7 | 439.6 ± 20.7 | 3.1 ± 0.2 | 17.4 ± 0.3 | 64.1 ± 1.5 | 9.8 ± 3.1 |
| II | 181.3 ± 7.5 | 498.7 ± 29.1 | 3.7 ± 0.3 | 16.8 ± 0.1 | 87.8 ± 2.1 | 15.1 ± 2.2 |
| III | 180.2 ± 7.2 | 458.6 ± 16.1 a | 3.3 ± 0.2 | 16.6 ± 0.2 | 88.7 ± 0.8 | 12.8 ± 4.8 a |
| IV | 179.7 ± 5.4 | 449.2 ± 26.2 b | 3.2 ± 0.3 a | 16.6 ± 0.1 | 87.4 ± 0.6 | 11.2 ± 2.5 b |
2.3. Effects of A. capillaris Extracts on Serum Lipoproteins Levels
| Groups | TC (mg/dL) | TG (mg/dL) | HDL-c (mg/dL) | LDL-c (mg/dL) | CRI | AI |
|---|---|---|---|---|---|---|
| I | 77.7 ± 10.5 | 77.7 ± 6.1 | 45.5 ± 4.1 | 16.7 ± 8.1 | 1.7 ± 0.2 | 0.4 ± 0.2 |
| II | 94.8 ± 6.1 c | 98.3 ± 12.4 c | 44.5 ± 4.3 | 33.6 ± 4.2 c | 2.3 ± 0.3 c | 0.8 ± 0.2 c |
| III | 83.0 ± 8.9 a | 87.7 ± 9.7 a | 48.2 ± 4.8 | 17.3 ± 5.4 a | 1.7 ± 0.1 a | 0.4 ± 0.1 b |
| IV | 82.0 ± 6.6 a | 81.9 ± 9.6 a | 47.9 ± 4.6 | 17.9 ± 4.8 a | 1.7 ± 0.2 a | 0.4 ± 0.3 b |
2.4. Effects of A. capillaris Extracts on HFD-Induced Fatty Liver
| Groups | Liver weight (g) | SOD (U/mg protein) | Serum (mg/dL) | Liver lipid (mg/g wet wt) | ||
|---|---|---|---|---|---|---|
| AST | ALT | TC | TG | |||
| I | 10.0 ± 0.6 | 2.42 ± 0.5 | 115.1 ± 11.6 | 62.1 ± 6.9 | 6.1 ± 0.7 | 37.2 ± 4.9 |
| II | 13.7 ± 0.8 c | 1.98 ± 0.7 | 144.4 ± 25.7 c | 84.5 ± 6.6 c | 10.1 ± 0.8 c | 64.8 ± 7.4 c |
| III | 10.7 ± 0.8 a | 2.41 ± 0.6 | 123.1 ± 16.4 a | 70.3 ± 4.9 b | 7.2 ± 0.6 a | 52.8 ± 6.8 a |
| IV | 10.2 ± 0.7 a | 2.42 ± 0.5 | 118.6 ± 10.5 b | 67.5 ± 6.8 b | 6.5 ± 0.5 a | 44.1 ± 5.8 a |
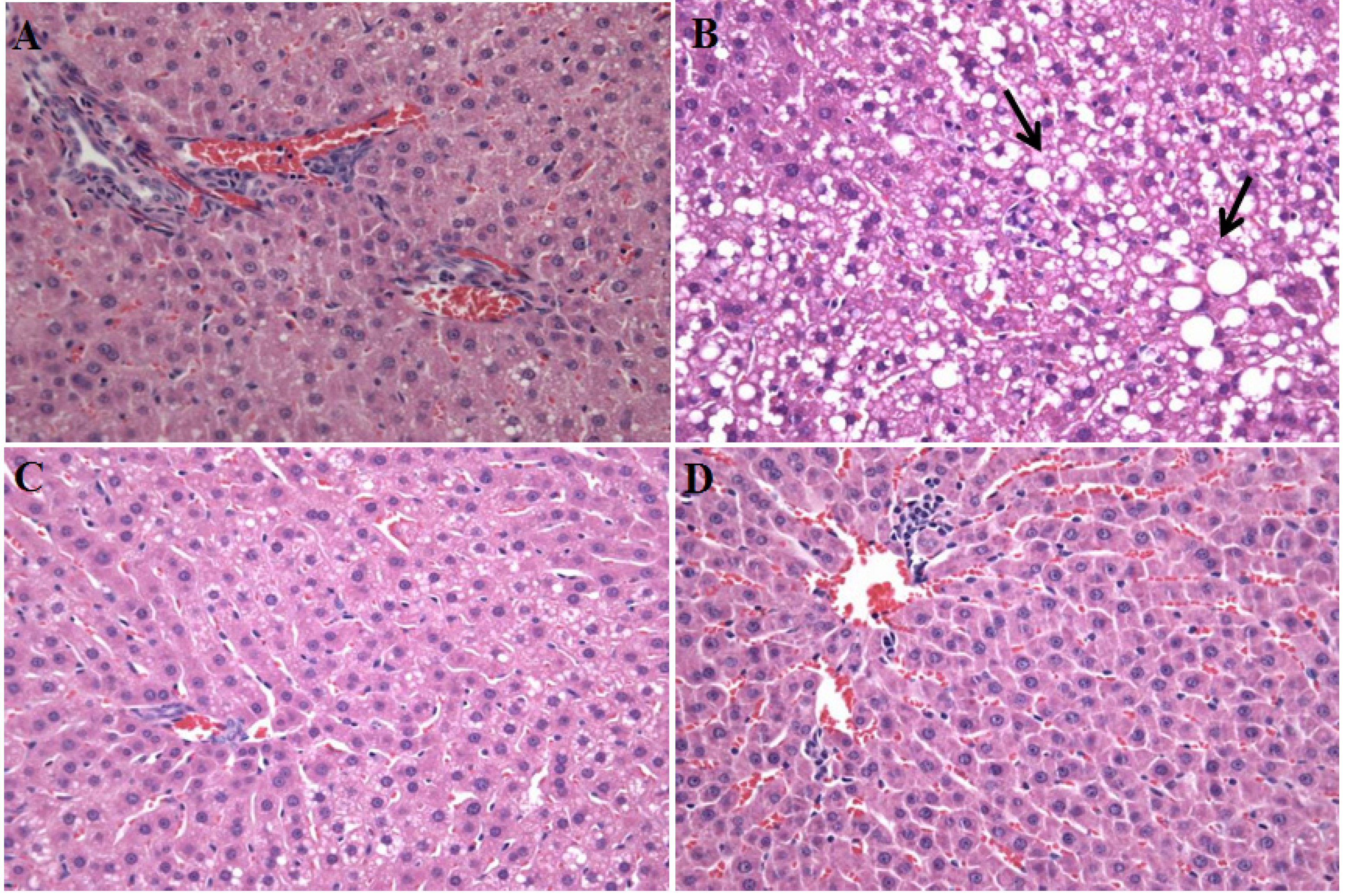
2.5. Discussion
3. Experimental
3.1. Determination of Scoparone in A. capillaris Extracts
3.2. TPC and Antioxidant Activity in A. capillaris Extracts
3.3. Animals and Diets
| Ingredients | Experimental Groups | |||
|---|---|---|---|---|
| ND | HFD | HFD mixed with A. capillaris | ||
| 0.40% | 0.80% | |||
| Casein | 20 | 20 | 20 | 20 |
| Sucrose | 10 | 10 | 10 | 10 |
| Corn starch | 39.75 | 24.75 | 24.35 | 23.95 |
| Soybean oil | 7 | 7 | 7 | 7 |
| Dextrose | 13.2 | 13.2 | 13.2 | 13.2 |
| Lard | - | 15 | 15 | 15 |
| Cellulose | 5 | 5 | 5 | 5 |
| L-cystine | 0.3 | 0.3 | 0.3 | 0.3 |
| AIN-mineral mixture | 3.5 | 3.5 | 3.5 | 3.5 |
| AIN-vitamin mixture | 1 | 1 | 1 | 1 |
| Choline bitrate | 0.25 | 0.25 | 0.25 | 0.25 |
| A. capillaris extracts | - | - | 0.4 | 0.8 |
| Total (%) | 100 | 100 | 100 | 100 |
| Energy (Kcal/100 g) | 380.2 | 487 | 487 | 487 |
3.4. Biochemical Parameter Analysis
3.5. Liver Histological Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Barness, L.A.; Opitz, J.M.; Gilbert-Barness, E. Obesity: genetic, molecular, and environmental aspects. Am. J. Med. Genet. Part A 2007, 143A, 3016–3034. [Google Scholar] [CrossRef]
- Powell, A.G.; Apovian, C.M.; Aronne, L.J. New drug targets for the treatment of obesity. Clin. Pharmacol. Ther. 2011, 90, 40–51. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature—a review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Lim, D.W.; Song, M.; Park, J.; Park, S.W.; Kim, N.H.; Gaire, B.P.; Choi, H.Y.; Kim, H. Anti-obesity effect of HT048, a herbal combination, in high fat diet-induced obese rats. Molecules 2012, 17, 14765–14777. [Google Scholar] [CrossRef]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kim, J.Y.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta medica 2005, 71, 186–190. [Google Scholar] [CrossRef]
- Twaij, H.A.; Al-Badr, A.A. Hypoglycemic activity of Artemisia herba alba. J. Ethnopharmacol. 1988, 24, 123–126. [Google Scholar] [CrossRef]
- Lee, J.; Chae, K.; Ha, J.; Park, B.Y.; Lee, H.S.; Jeong, S.; Kim, M.Y.; Yoon, M. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J. Ethnopharmacol. 2008, 115, 263–270. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Liu, H.; Qiu, Z.; Chen, L. Effects of Artemisia capillaris on blood glucose and lipid in mice. Zhong yao cai 1998, 21, 408–411. [Google Scholar]
- Kim, E.K.; Kwon, K.B.; Han, M.J.; Song, M.Y.; Lee, J.H.; Lv, N.; Choi, K.B.; Ryu, D.G.; Kim, K.S.; Park, J.W.; et al. Inhibitory effect of Artemisia capillaris extract on cytokine-induced nitric oxide formation and cytotoxicity of RINm5F cells. Int. J. Mol. Med. 2007, 19, 535–540. [Google Scholar]
- Kim, Y.S.; Bahn, K.N.; Hah, C.K.; Gang, H.I.; Ha, Y.L. Inhibition of 7,12-dimethylbenz[a]anthracene induced mouse skin carcinogenesis by Artemisia capillaris. J. Food Sci. 2008, 73, T16–T20. [Google Scholar]
- Han, J.M.; Kim, H.G.; Choi, M.K.; Lee, J.S.; Lee, J.S.; Wang, J.H.; Park, H.J.; Son, S.W.; Hwang, S.Y.; Son, C.G. Artemisia capillaris extract protects against bile duct ligation-induced liver fibrosis in rats. Exp. Toxicol. Pathol. 2013, 65, 837–844. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chang, H.H.; Chen, J.H.; Hsueh, M.L.; Kuo, J.J. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J. Ethnopharmacol. 2007, 109, 318–324. [Google Scholar]
- Wang, J.H.; Choi, M.K.; Shin, J.W.; Hwang, S.Y.; Son, C.G. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J. Ethnopharmacol. 2012, 140, 179–185. [Google Scholar] [CrossRef]
- Atmaca, M.; Bilgin, H.M.; Obay, B.D.; Diken, H.; Kelle, M.; Kale, E. The hepatoprotective effect of coumarin and coumarin derivates on carbon tetrachloride-induced hepatic injury by antioxidative activities in rats. J. Physiol. Biochem. 2011, 67, 569–576. [Google Scholar] [CrossRef]
- Huang, H.C.; Chu, S.H.; Chao, P.D. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur. J. Pharmacol. 1991, 198, 211–213. [Google Scholar] [CrossRef]
- Huang, H.C.; Lee, C.R.; Weng, Y.I.; Lee, M.C.; Lee, Y.T. Vasodilator effect of scoparone (6,7-dimethoxycoumarin) from a Chinese herb. Eur. J. Pharmacol. 1992, 218, 123–128. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, K.B.; Lee, J.H.; Park, B.H.; Park, J.W.; Lee, H.K.; Jhee, E.C.; Yang, J.Y. Inhibition of cytokine-mediated nitric oxide synthase expression in rat insulinoma cells by scoparone. Biol. Pharm. Bull. 2007, 30, 242–246. [Google Scholar] [CrossRef]
- Grujic, N.; Lepojevic, Z.; Srdjenovic, B.; Vladic, J.; Sudji, J. Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts. Molecules 2012, 17, 2518–2528. [Google Scholar] [CrossRef]
- Sourivong, P.; Schronerova, K.; Babincova, M. Scoparone inhibits ultraviolet radiation-induced lipid peroxidation. Z. Naturforsch. C 2007, 62, 61–64. [Google Scholar]
- Huang, H.C.; Weng, Y.I.; Lee, C.R.; Jan, T.R.; Chen, Y.L.; Lee, Y.T. Protection by scoparone against the alterations of plasma lipoproteins, vascular morphology and vascular reactivity in hyperlipidaemic diabetic rabbit. Brit. J. Pharmacol. 1993, 110, 1508–1514. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid. Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLos Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Loncar, D.; Afzelius, B.A.; Cannon, B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J. Ultrastruct. Mol. Struct. Res. 1988, 101, 109–122. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996, 312, 478–481. [Google Scholar] [CrossRef]
- Haque, M.; Sanyal, A.J. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best. Pract. Res. Clin. Gastroenterol. 2002, 16, 709–731. [Google Scholar] [CrossRef]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver. Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef]
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition 2006, 22, 956–964. [Google Scholar] [CrossRef]
- Shertzer, H.G.; Schneider, S.N.; Kendig, E.L.; Clegg, D.J.; D'Alessio, D.A.; Genter, M.B. Acetaminophen normalizes glucose homeostasis in mouse models for diabetes. Biochem. Pharmacol. 2008, 75, 1402–1410. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 2003, 112, 91–100. [Google Scholar] [Green Version]
- Milagro, F.I.; Campion, J.; Martinez, J.A. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 2006, 14, 1118–1123. [Google Scholar] [CrossRef]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
- Kayano, S.; Kikuzaki, H.; Ikami, T.; Suzuki, T.; Mitani, T.; Nakatani, N. A new bipyrrole and some phenolic constituents in prunes (Prunus domestica L.) and their oxygen radical absorbance capacity (ORAC). Biosci. Biotechnol. Biochem. 2004, 68, 942–944. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Abbott, R.D.; Wilson, P.W.; Kannel, W.B.; Castelli, W.P. High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis 1988, 8, 207–211. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Adeyemi, O.O.; Agbaje, E.O. Anti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. J. Ethnopharmacol. 2010, 130, 307–314. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Sample Availability: A. capillaris extracts are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, D.W.; Kim, Y.T.; Jang, Y.-J.; Kim, Y.-E.; Han, D. Anti-Obesity Effect of Artemisia capillaris Extracts in High-Fat Diet-Induced Obese Rats. Molecules 2013, 18, 9241-9252. https://doi.org/10.3390/molecules18089241
Lim DW, Kim YT, Jang Y-J, Kim Y-E, Han D. Anti-Obesity Effect of Artemisia capillaris Extracts in High-Fat Diet-Induced Obese Rats. Molecules. 2013; 18(8):9241-9252. https://doi.org/10.3390/molecules18089241
Chicago/Turabian StyleLim, Dong Wook, Yun Tai Kim, Yu-Jung Jang, Young-Eon Kim, and Daeseok Han. 2013. "Anti-Obesity Effect of Artemisia capillaris Extracts in High-Fat Diet-Induced Obese Rats" Molecules 18, no. 8: 9241-9252. https://doi.org/10.3390/molecules18089241




