Reactive Oxygen Species Mediate Isoalantolactone-Induced Apoptosis in Human Prostate Cancer Cells
Abstract
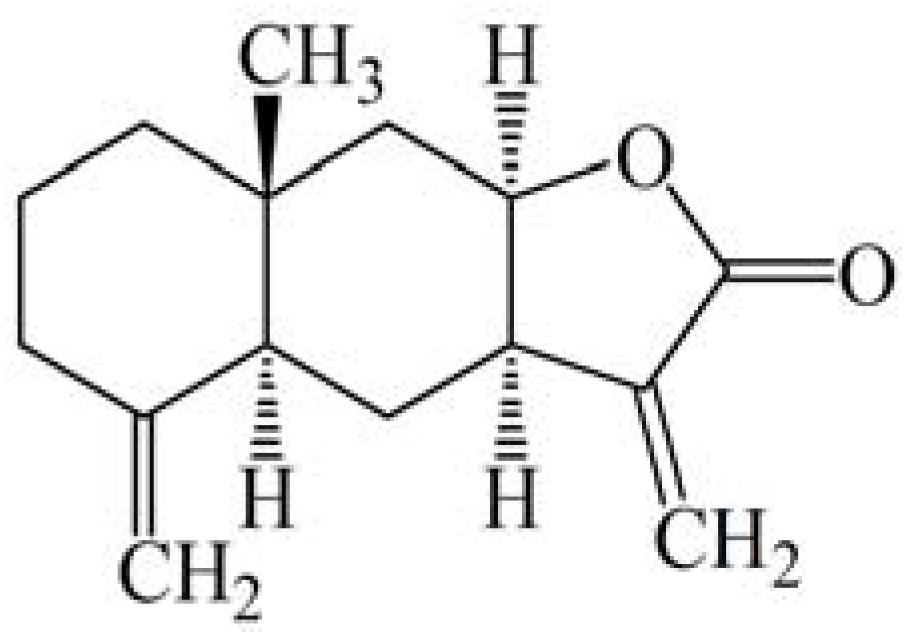
:1. Introduction
2. Results and Discussion
2.1. Antiproliferative Effects of Isoalantolactone in Prostate Cancer Cells
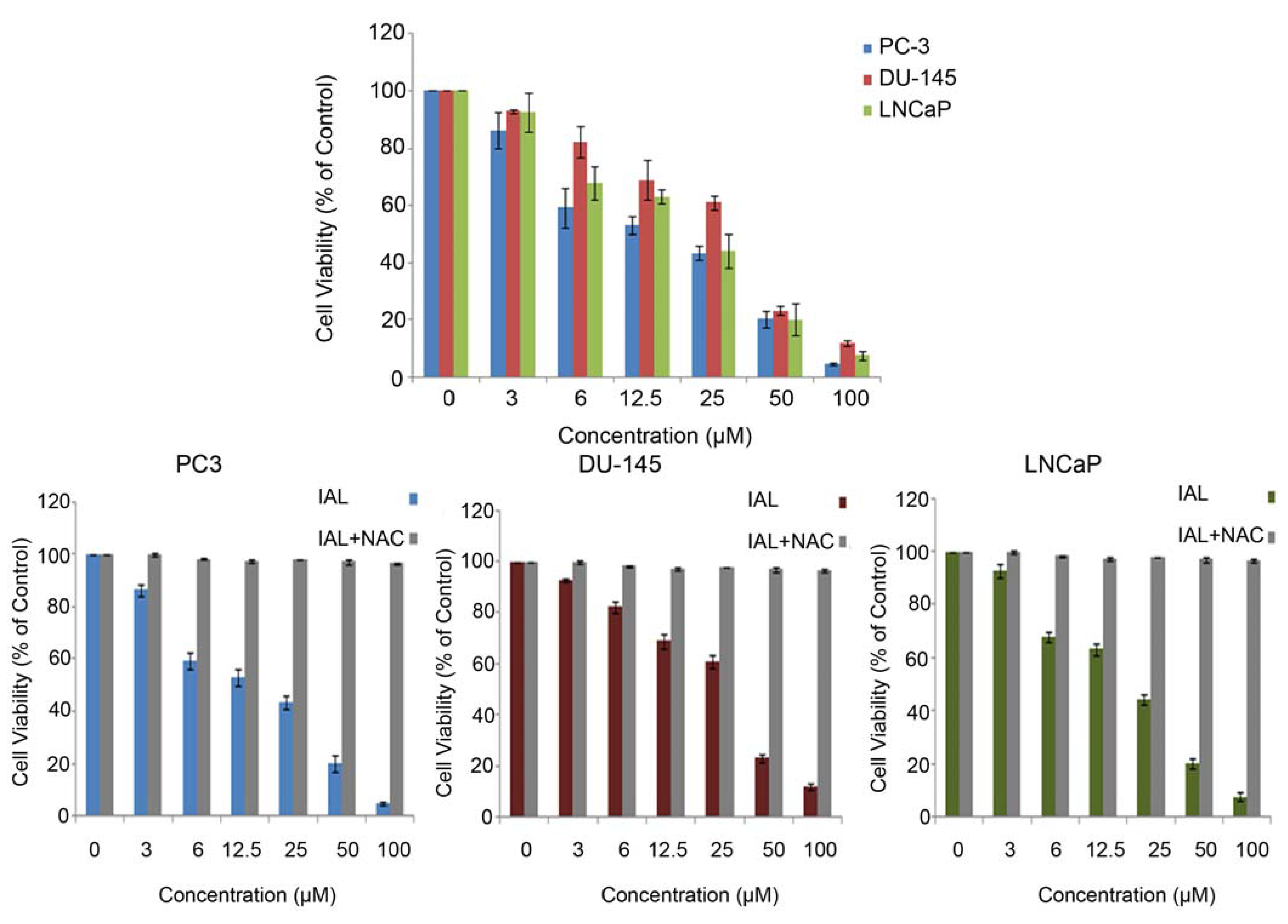
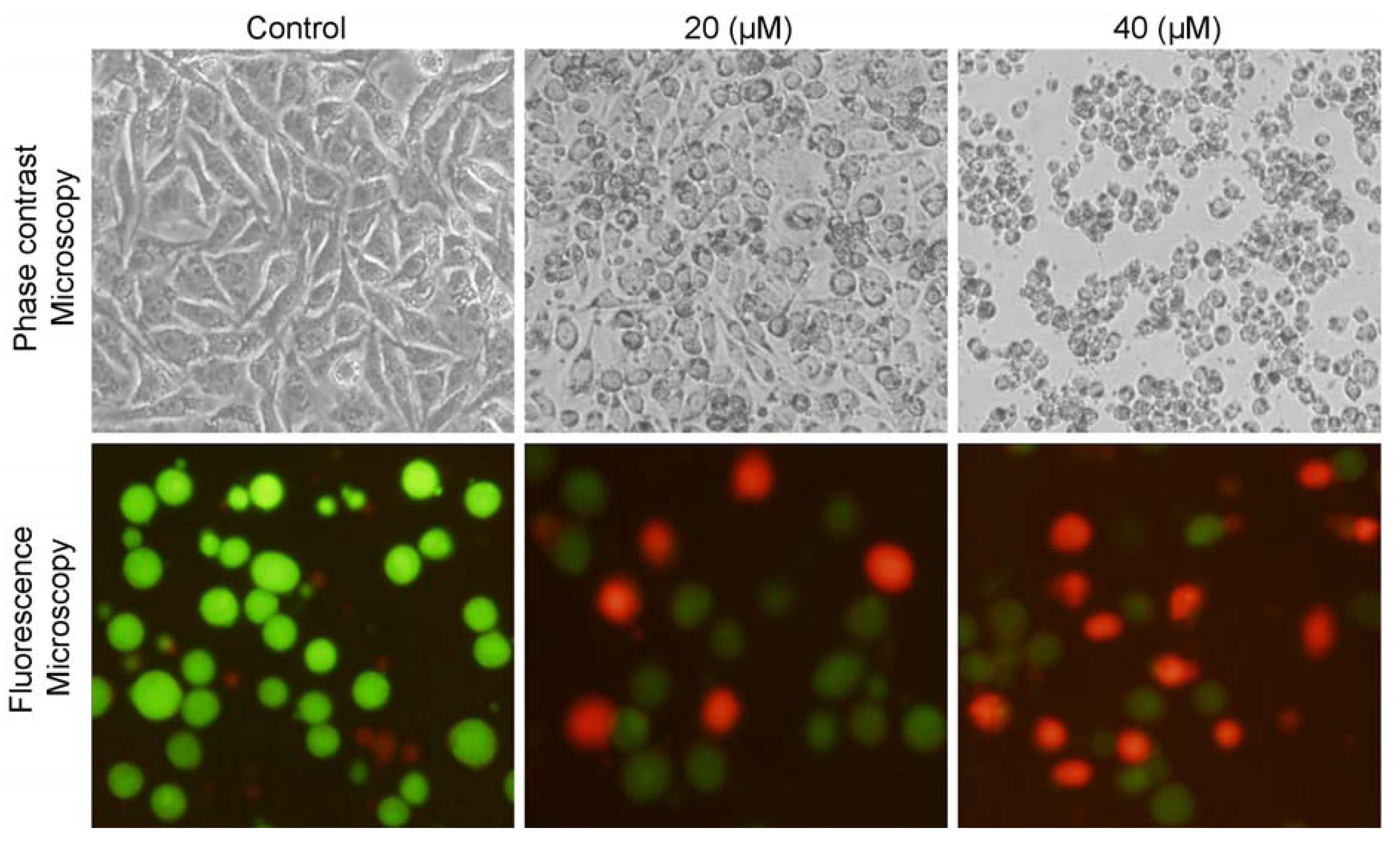
2.2. Isoalantolactone Induced Morphological Changes and Cell Death in Prostate Cancer Cells
2.3. Isoalantolactone Induced G2/M Cell Cycle Arrest in PC3 Cells
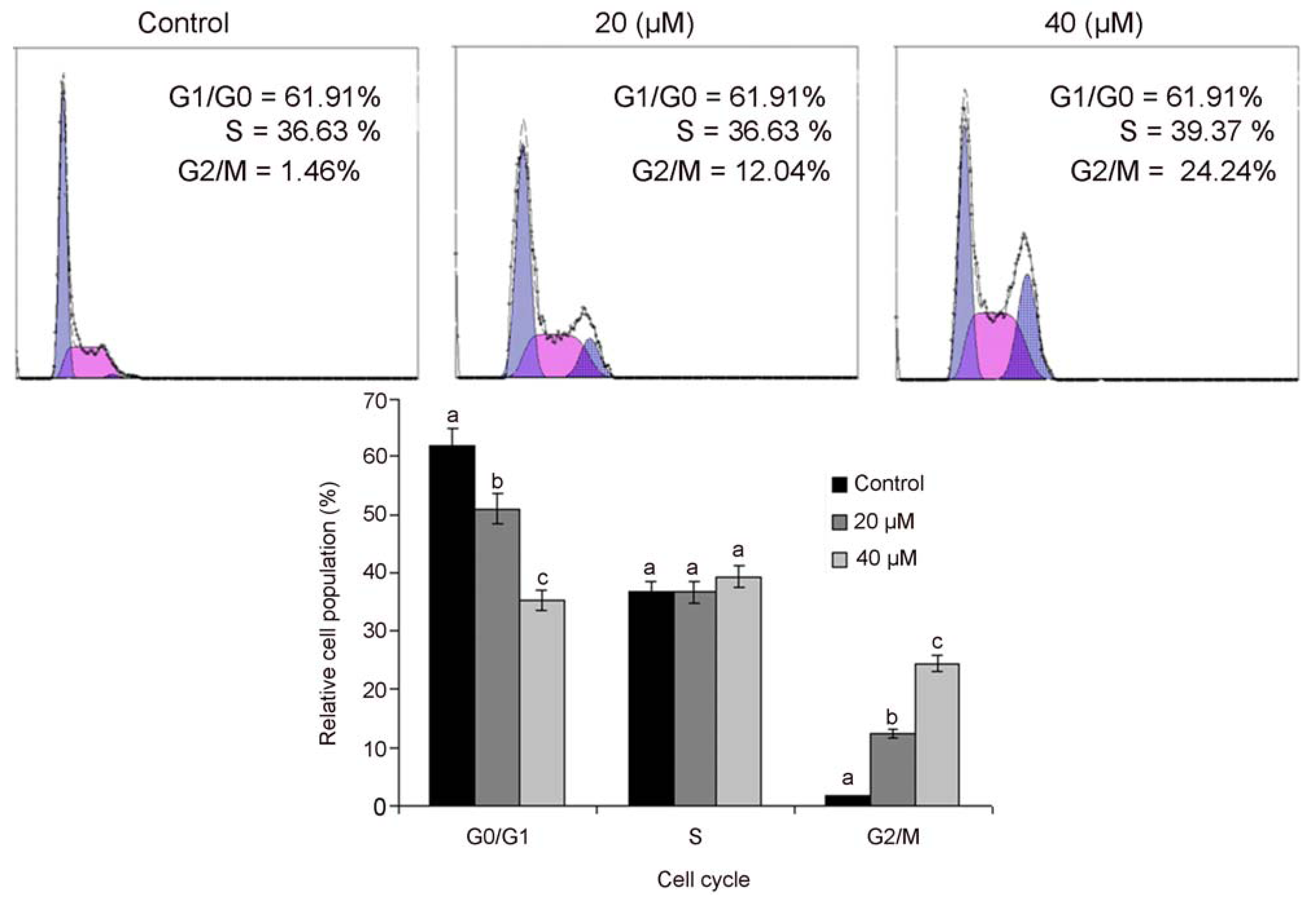
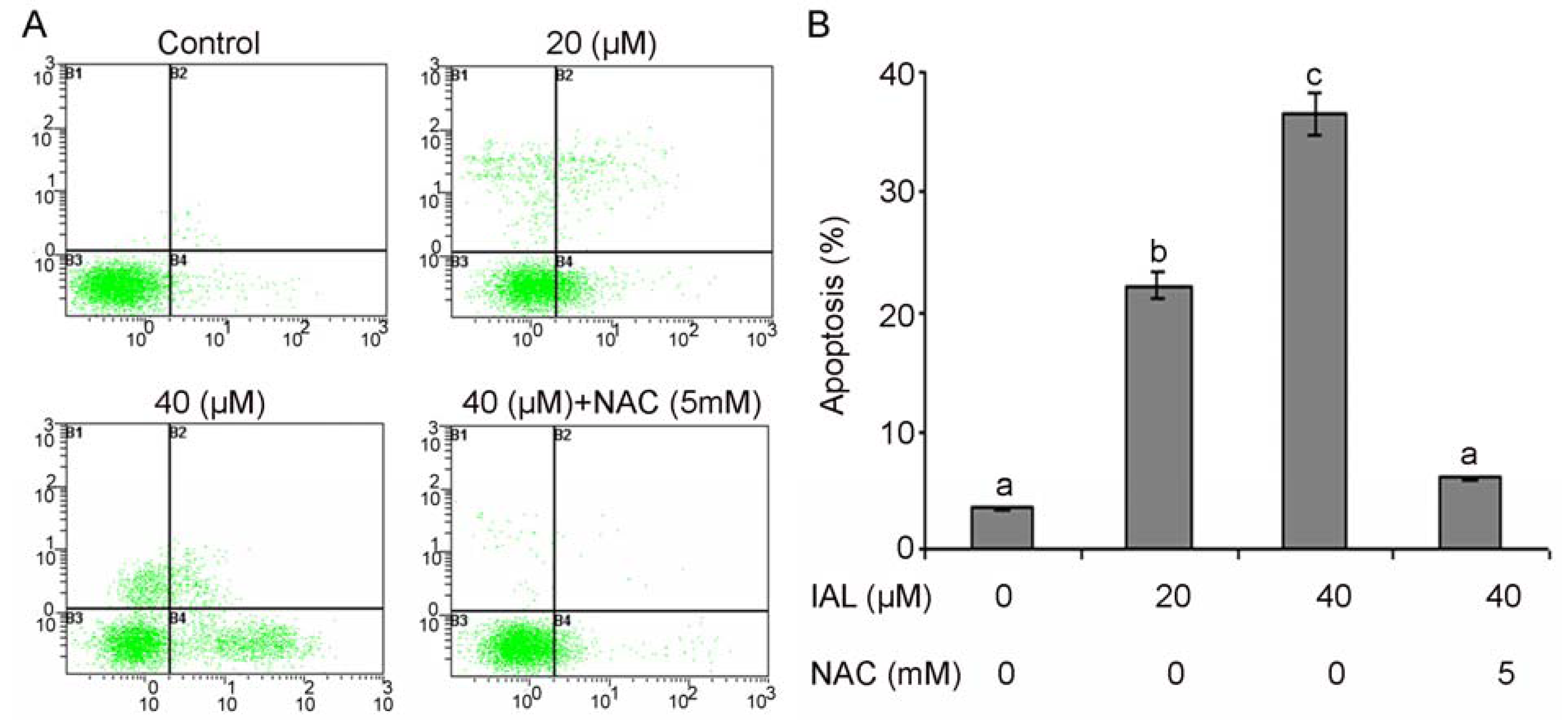
2.4. Effect of Isoalantolactone on Apoptosis in Human Prostate Cancer Cells
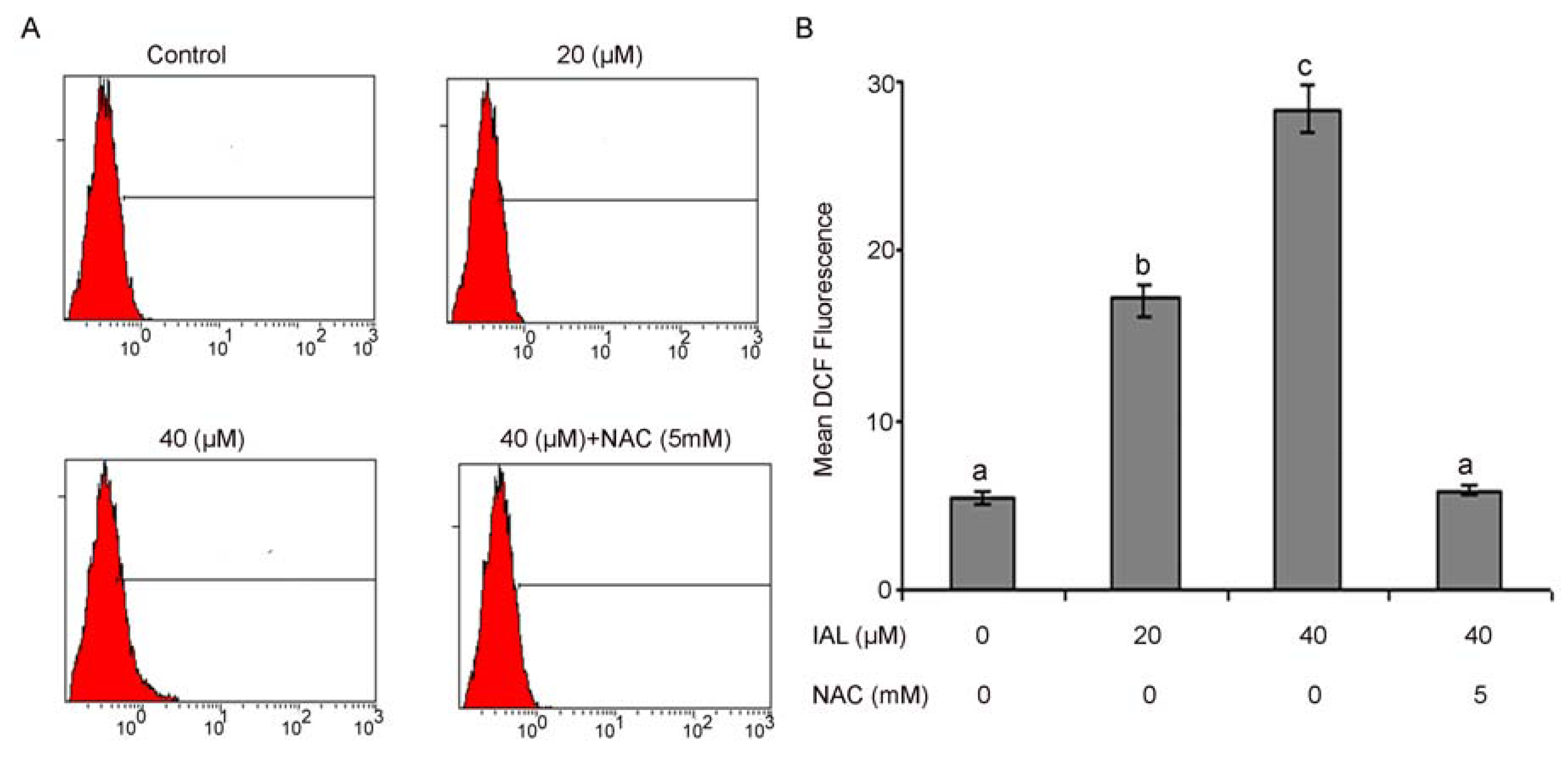
2.5. Isoalantolactone Promoted ROS Generation in Prostate Cancer Cells
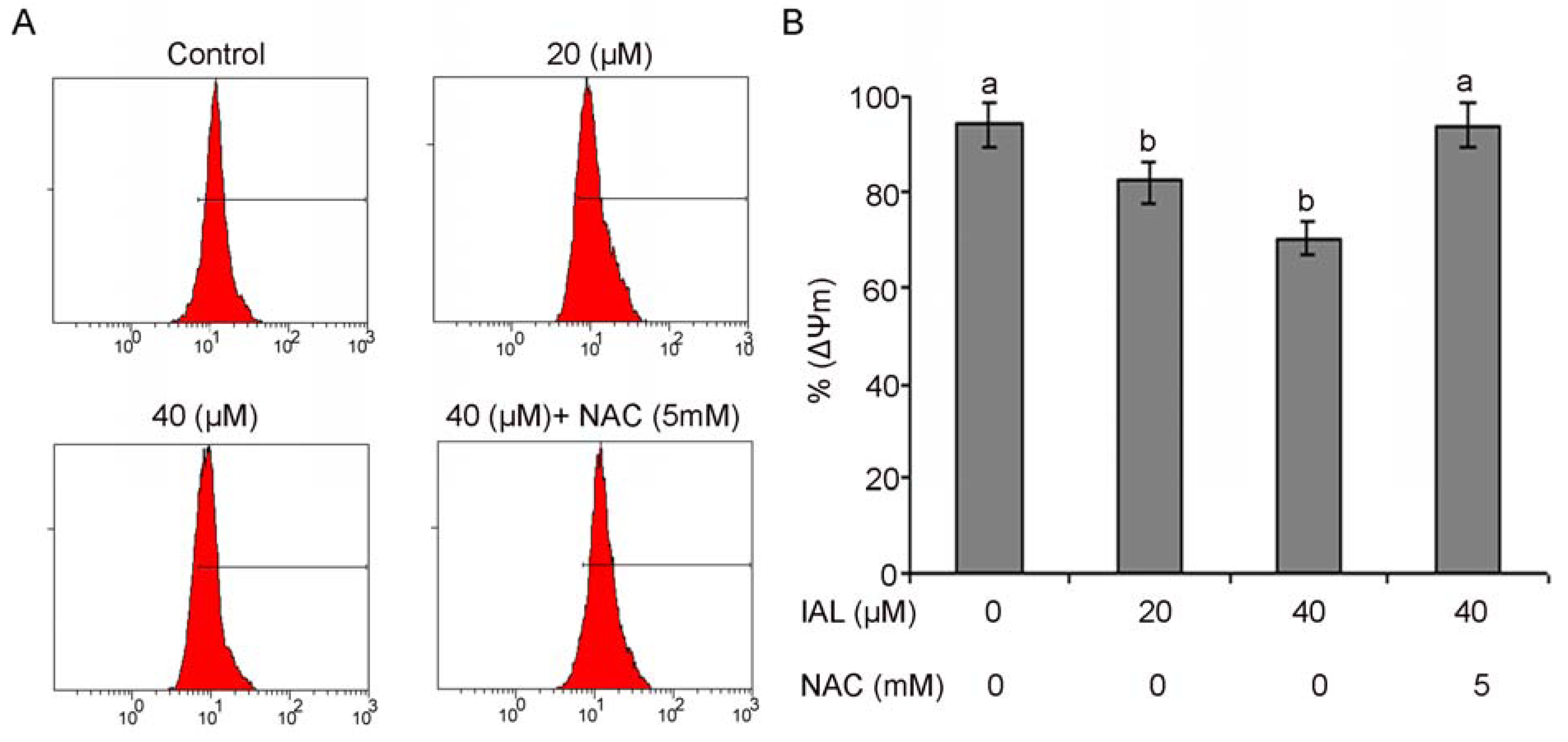
2.6. Isoalantolactone Decreased Mitochondrial Membrane Potential in PC3 Cells
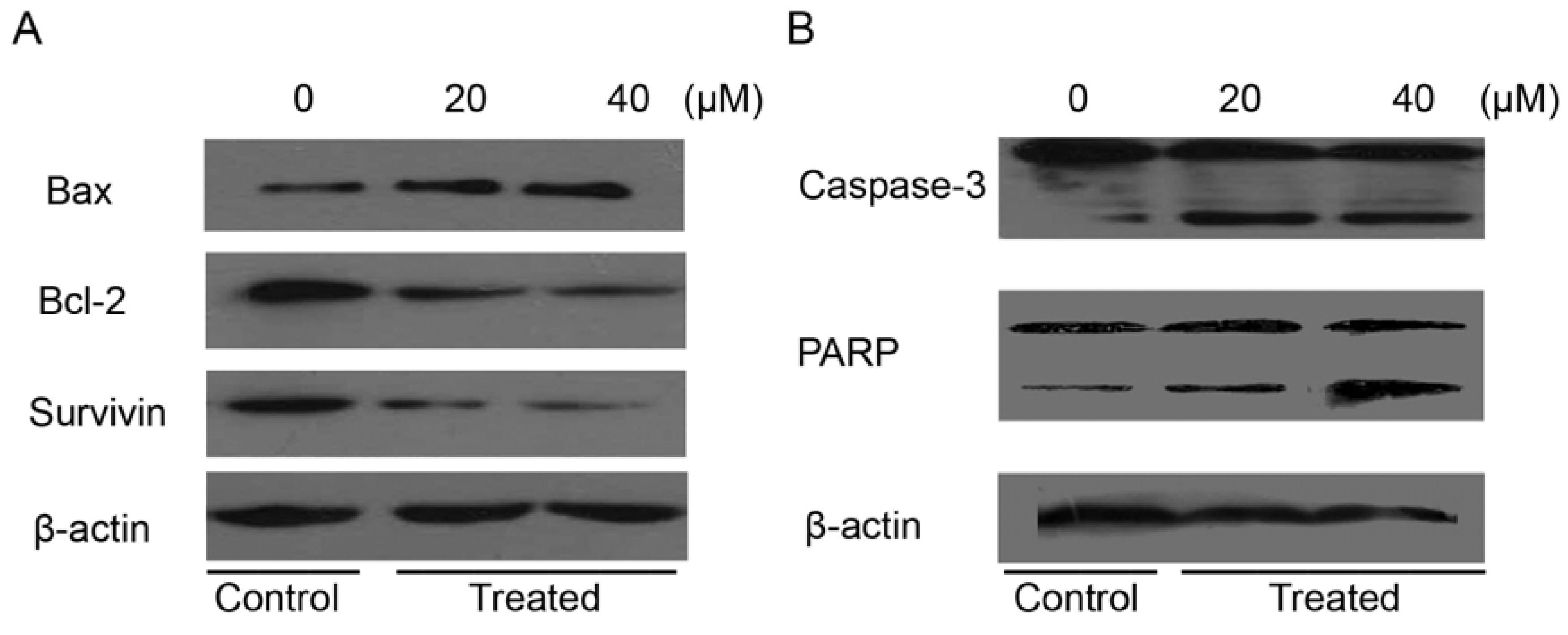
2.7. Isoalantolactone Regulated Apoptosis-Related Proteins in Prostate Cancer Cells
3. Experimental
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell Proliferation Assay
3.4. Morphological Observation under Phase Contrast and Fluorescence Microscope
3.5. Flow Cytometric Analysis of Cell Cycle
3.6. Flow Cytometric Determination of Apoptosis
3.7. Flow Cytometric Determination of Reactive Oxygen Species (ROS) in PC3 Cells
3.8. Flow Cytometric Determination of Mitochondrial Membrane Potential (ΔΨm)
3.9. Western Blotting
3.10. Statistical Analysis of Data
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts and Figures 2013. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf (accessed on 26 July 2013).
- Sim, H.G.; Cheng, C.W. Changing demography of prostate cancer in Asia. Eur. J. Cancer 2005, 41, 834–845. [Google Scholar]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar]
- Fruehauf, J.P.; Meyskens, F.L., Jr. Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar]
- Gu, J.Q.; Gills, J.J.; Park, E.J.; Mata-Greenwood, E.; Hawthorne, M.E.; Axelrod, F.; Chavez, P.I.; Fong, H.H.; Mehta, R.G.; Pezzuto, J.M.; et al. Sesquiterpenoids from Tithonia diversifolia with potential cancer chemopreventive activity. J. Nat. Prod. 2002, 65, 532–536. [Google Scholar]
- Koch, E.; Klaas, C.A.; Rungeler, P.; Castro, V.; Mora, G.; Vichnewski, W.; Merfort, I. Inhibition of inflammatory cytokine production and lymphocyte proliferation by structurally different sesquiterpene lactones correlates with their effect on activation of NF-kappaB. Biochem. Pharmacol. 2001, 62, 795–801. [Google Scholar]
- Tan, R.X.; Tang, H.Q.; Hu, J.; Shuai, B. Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry 1998, 49, 157–161. [Google Scholar]
- Tripathi, Y.B.; Tripathi, P.; Upadhyay, B.N. Assessment of the adrenergic beta-blocking activity of Inula racemosa. J. Ethnopharmacol. 1988, 23, 3–9. [Google Scholar]
- Konishi, T.; Shimada, Y.; Nagao, T.; Okabe, H.; Konoshima, T. Antiproliferative sesquiterpene lactones from the roots of Inula helenium. Biol. Pharm. Bull. 2002, 25, 1370–1372. [Google Scholar]
- Pal, H.C.; Sehar, I.; Bhushan, S.; Gupta, B.D.; Saxena, A.K. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. In Vitro 2010, 24, 1599–1609. [Google Scholar]
- Khan, M.; Ding, C.; Rasul, A.; Yi, F.; Li, T.; Gao, H.; Gao, R.; Zhong, L.; Zhang, K.; Fang, X.; Ma, T. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int. J. Biol. Sci. 2012, 8, 533–547. [Google Scholar]
- Collins, K.; Jacks, T.; Pavletich, N.P. The cell cycle and cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 2776–2778. [Google Scholar]
- Grana, X.; Reddy, E.P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar]
- Rasul, A.; Ding, C.; Li, X.; Khan, M.; Yi, F.; Ali, M.; Ma, T. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis 2012, 17, 1104–1119. [Google Scholar]
- Rasul, A.; Yu, B.; Khan, M.; Zhang, K.; Iqbal, F.; Ma, T.; Yang, H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012, 40, 1153–1161. [Google Scholar]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar]
- Leist, M.; Jaattela, M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar]
- Pommier, Y.; Sordet, O.; Antony, S.; Hayward, R.L.; Kohn, K.W. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene 2004, 23, 2934–2949. [Google Scholar] [CrossRef]
- Fulda, S. Evasion of apoptosis as a cellular stress response in cancer. Int. J. Cell Biol. 2010, 2010, 370835. [Google Scholar]
- Lawen, A. Apoptosis-an introduction. Bioessays 2003, 25, 888–896. [Google Scholar]
- Reed, J.C. Apoptosis-based therapies. Nat. Rev. Drug Discov. 2002, 1, 111–121. [Google Scholar]
- Khan, M.; Li, T.; Ahmad Khan, M.K.; Rasul, A.; Nawaz, F.; Sun, M.; Zheng, Y.; Ma, T. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Slater, A.F.; Stefan, C.; Nobel, I.; van den Dobbelsteen, D.J.; Orrenius, S. echanisms and oxidative stress in apoptosis. Toxicol. Lett. 1995, 82–83, 149–153. [Google Scholar]
- Lee, J.H.; Baek, N.I.; Kim, S.H.; Park, H.W.; Yang, J.H.; Lee, J.J.; Kim, S.J.; Jeong, S.; Oh, C.H.; Lee, K.H.; Kim, D.K. A new cytotoxic prenylated chalcone from Sophora flavescens. Arch. Pharm. Res. 2007, 30, 408–411. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Kowaltowski, A.J.; Grijalba, M.T.; Meinicke, A.R.; Castilho, R.F. The role of reactive oxygen species in mitochondrial permeability transition. Biosci. Rep. 1997, 17, 43–52. [Google Scholar]
- Wan, X.S.; Zhou, Z.; Kennedy, A.R. Adaptation of the dichlorofluorescein assay for detection of radiation-induced oxidative stress in cultured cells. Radiat. Res. 2003, 160, 622–630. [Google Scholar] [CrossRef]
- Rasul, A.; Bao, R.; Malhi, M.; Zhao, B.; Tsuji, I.; Li, J.; Li, X. Induction of Apoptosis by Costunolide in Bladder Cancer Cells is Mediated through ROS Generation and Mitochondrial Dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar]
- Mallat, Z.; Tedgui, A. Apoptosis in the vasculature: mechanisms and functional importance. Br. J. Pharmacol. 2000, 130, 947–962. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar]
- Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell Mol. Med. 2003, 7, 249–257. [Google Scholar]
- Danial, N.N. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007, 13, 7254–7263. [Google Scholar] [CrossRef]
- Zhang, M.; Latham, D.E.; Delaney, M.A.; Chakravarti, A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene 2005, 24, 2474–2482. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar]
- Adams, J.M. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003, 17, 2481–2495. [Google Scholar] [CrossRef]
- Rasul, A.; Yu, B.; Yang, L.; Arshad, M.; Khan, M.; Ma, T.; Yang, H. Costunolide, a sesquiterpene lactone induces G2/M phase arrest and mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Med. Plant Res. 2012, 6, 1191–1200. [Google Scholar]
- Rasul, A.; Yu, B.; Yang, L.F.; Ali, M.; Khan, M.; Ma, T.; Yang, H. Induction of mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells by kuraridin and Nor-kurarinone isolated from Sophora flavescens. Asian. Pac. J. Cancer Prev. 2011, 12, 2499–2504. [Google Scholar]
- Sample Availability: Samples of the isoalantolactone is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rasul, A.; Di, J.; Millimouno, F.M.; Malhi, M.; Tsuji, I.; Ali, M.; Li, J.; Li, X. Reactive Oxygen Species Mediate Isoalantolactone-Induced Apoptosis in Human Prostate Cancer Cells. Molecules 2013, 18, 9382-9396. https://doi.org/10.3390/molecules18089382
Rasul A, Di J, Millimouno FM, Malhi M, Tsuji I, Ali M, Li J, Li X. Reactive Oxygen Species Mediate Isoalantolactone-Induced Apoptosis in Human Prostate Cancer Cells. Molecules. 2013; 18(8):9382-9396. https://doi.org/10.3390/molecules18089382
Chicago/Turabian StyleRasul, Azhar, Jun Di, Faya Martin Millimouno, Mahadev Malhi, Ichiro Tsuji, Muhammad Ali, Jiang Li, and Xiaomeng Li. 2013. "Reactive Oxygen Species Mediate Isoalantolactone-Induced Apoptosis in Human Prostate Cancer Cells" Molecules 18, no. 8: 9382-9396. https://doi.org/10.3390/molecules18089382




