A Facile Fabrication of Alginate Microbubbles Using a Gas Foaming Reaction
Abstract
:1. Introduction
2. Results and Discussion
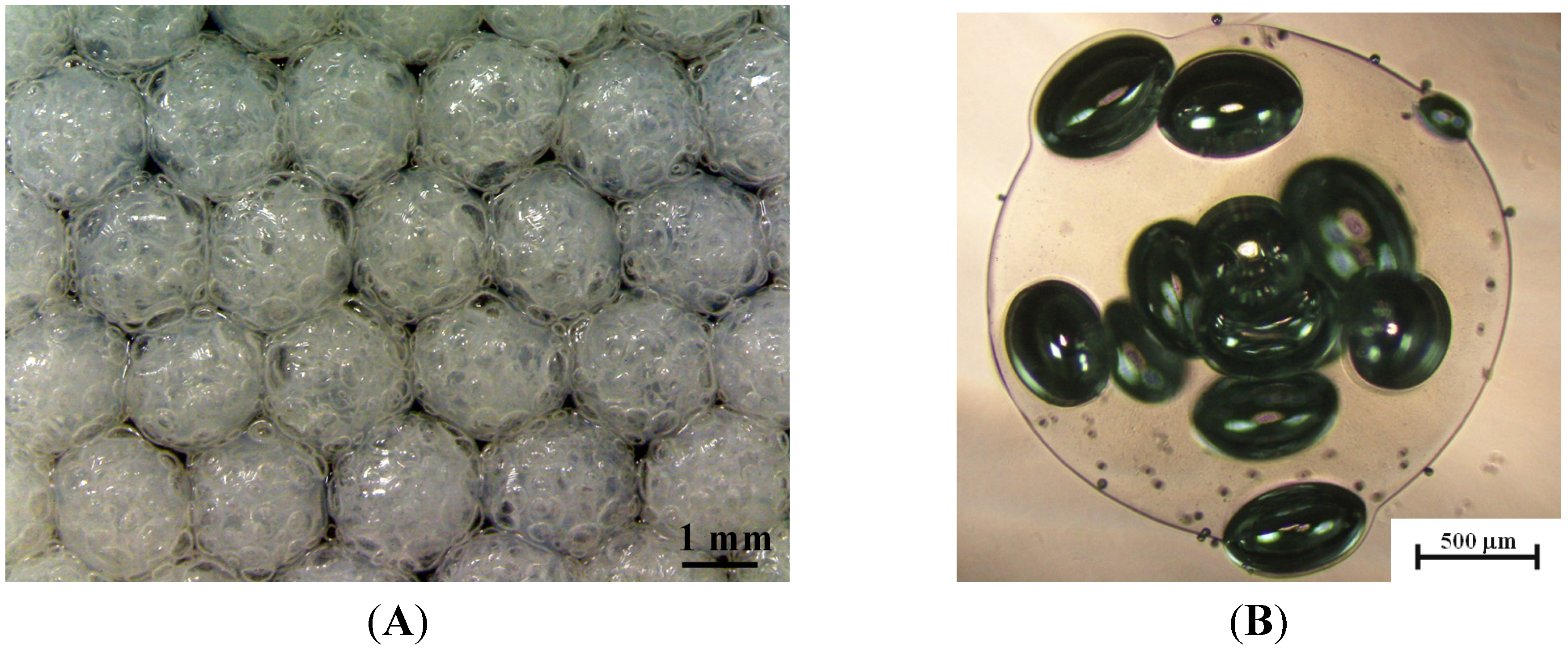
2.1. Ca-Alginate Microbubbles
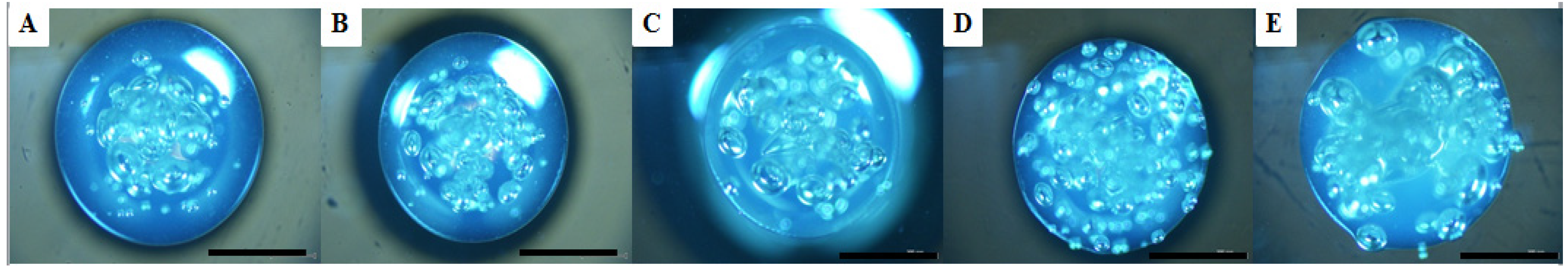
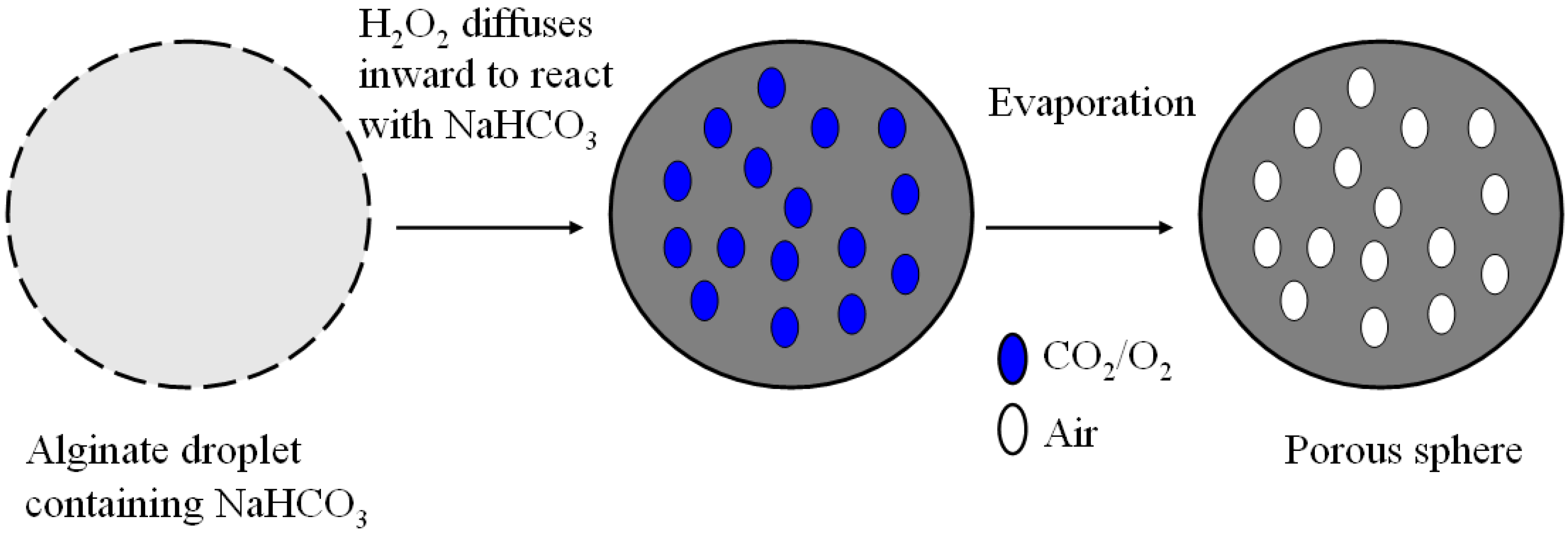
2.2. Microbubble Evolution

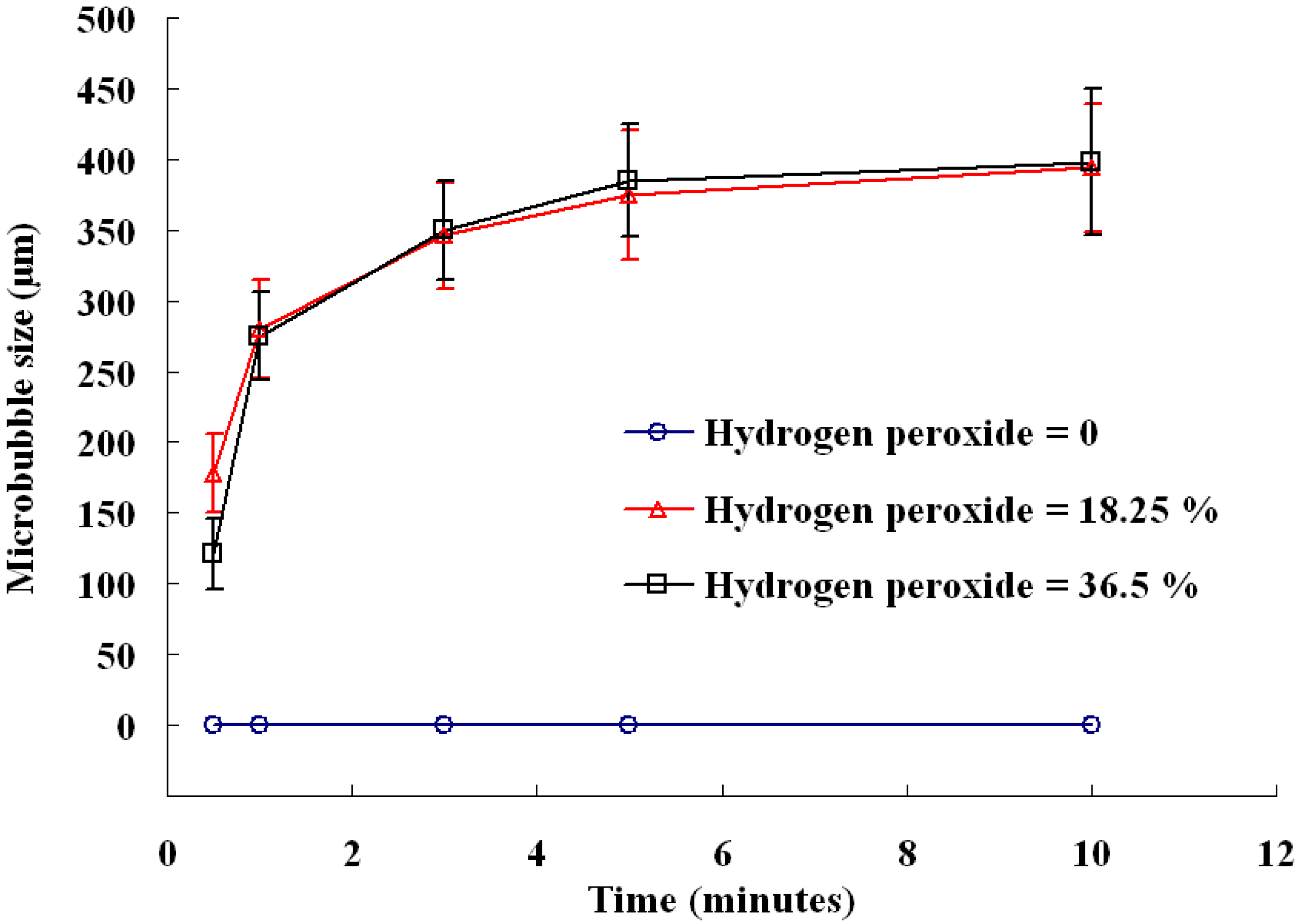
2.3. Influence of Reactant Concentration

3. Experimental
3.1. Materials
3.2. Preparation of Ca-Alginate Particles
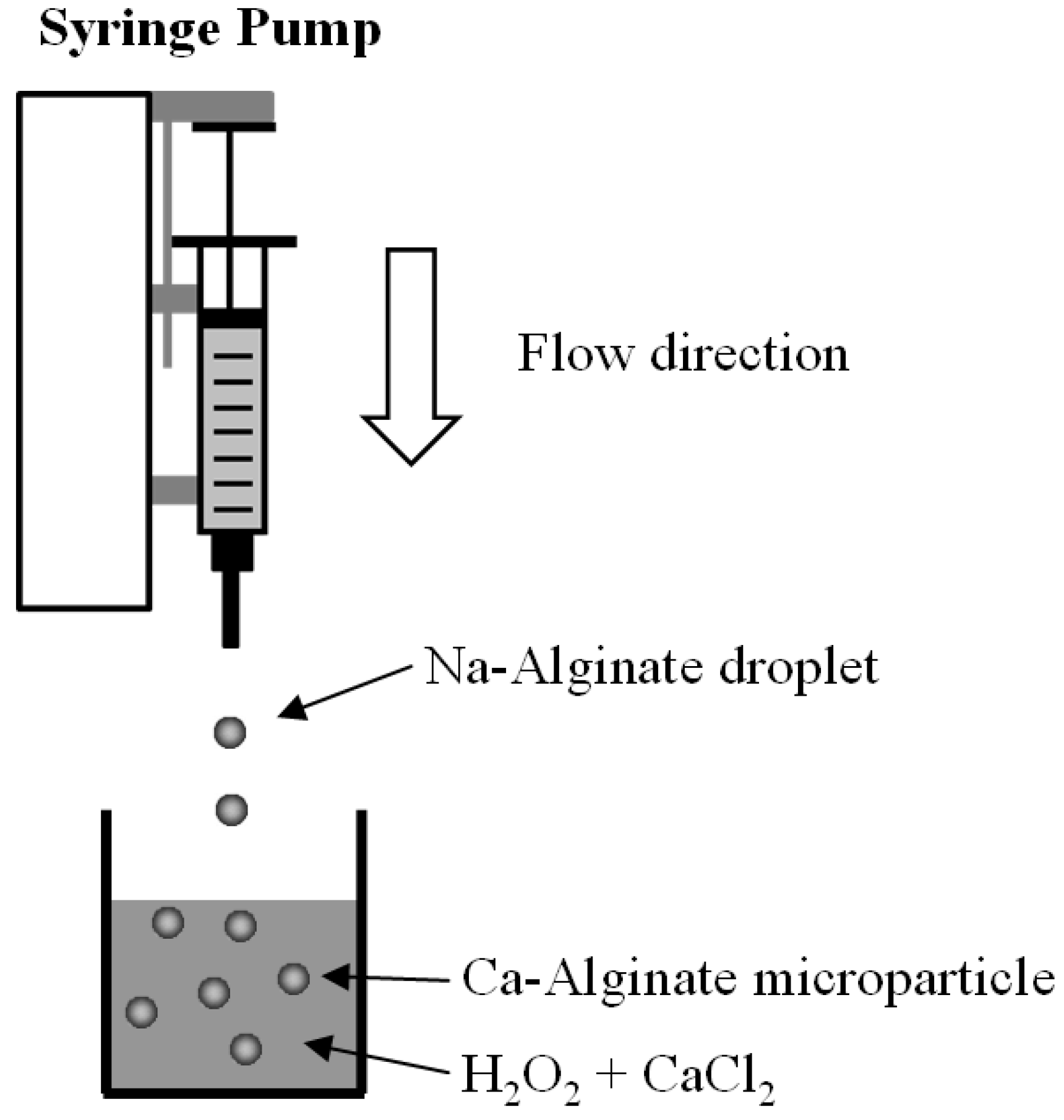
3.3. Preparation of Ca-Alginate Microbubbles
3.4. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, T.K.; Yoon, J.J.; Lee, D.S.; Park, T.G. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials 2006, 27, 152–159. [Google Scholar] [CrossRef]
- Dressaire, E.; Bee, R.; Bell, D.C.; Lips, A.; Stone, H.A. Interfacial polygonal nanopatterning of stable microbubbles. Science 2008, 320, 1198–1201. [Google Scholar] [CrossRef]
- Capece, S.; Chiessi, E.; Cavalli, R.; Giustetto, P.; Grishenkov, D.; Paradossi, G. A general strategy for obtaining biodegradable polymer shelled microbubbles as theranostic devices. Chem. Commun. 2013, 49, 5763–5765. [Google Scholar]
- Hosny, N.A.; Mohamedi, G.; Rademeyer, P.; Owen, J.; Wu, Y.; Tang, M.X.; Eckersley, R.J.; Stride, E.; Kuimova, M.K. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl. Acad. Sci. USA 2013, 110, 9225–9230. [Google Scholar] [CrossRef]
- Geers, B.; de Wever, O.; Demeester, J.; Bracke, M.; de Smedt, S.C.; Lentacker, I. Targeted liposome-loaded microbubbles for cell-specific ultrasound-triggered drug delivery. Small 2013. [Google Scholar] [CrossRef]
- Noble, M.L.; Kuhr, C.S.; Graves, S.S.; Loeb, K.R.; Sun, S.S.; Keilman, G.W.; Morrison, K.P.; Paun, M.; Storb, R.F.; Miao, C.H. Ultrasound-targeted microbubble destruction-mediated gene delivery into canine livers. Mol. Ther. 2013. [Google Scholar] [CrossRef]
- Villa, R.; Cerroni, B.; Viganò, L.; Margheritelli, S.; Abolafio, G.; Oddo, L.; Paradossi, G.; Zaffaroni, N. Targeted doxorubicin delivery by chitosan-galactosylated modified polymer microbubbles to hepatocarcinoma cells. Colloids Surf. B Biointerfaces 2013, 110, 434–442. [Google Scholar] [CrossRef]
- Bae, S.E.; Son, J.S.; Park, K.; Han, D.K. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J. Control. Release 2009, 133, 37–43. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, X.; Stride, E.; Huang, J.; Edirisinghe, M.; Schroeder, C.; Best, S.; Cameron, R.; Waller, D.; Donald, A. Novel preparation and characterization of porous alginate films. Carbohyd. Polym. 2010, 79, 989–997. [Google Scholar] [CrossRef]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of porous substrates: A review of processes using pore forming agents in the biomaterial field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef]
- Cui, W.; Bei, J.; Wang, S.; Zhi, G.; Zhao, Y.; Zhou, X.; Zhang, H.; Xu, Y. Preparation and evaluation of poly(L-lactide-co-glycolide) (PLGA) microbubbles as a contrast agent for myocardial contrast echocardiography. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 171–178. [Google Scholar]
- Zhang, H.; Ju, X.J.; Xie, R.; Cheng, C.J.; Ren, P.W.; Chu, L.Y. A microfluidic approach to fabricate monodisperse hollow or porous poly(HEMA-MMA) microspheres using single emulsions as templates. J. Colloid Interface Sci. 2009, 336, 235–243. [Google Scholar] [CrossRef]
- Butler, R.; Davies, C.M.; Cooper, A.I. Emulsion templating using high internal phase supercritical fluid emulsions. Adv. Mater. 2001, 13, 1459–1463. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef]
- Stride, E.; Edirisinghe, M. Novel microbubble preparation technologies. Soft Matter 2008, 4, 2350–2359. [Google Scholar] [CrossRef]
- Stride, E.; Edirisinghe, M. Novel preparation techniques for controlling microbubble uniformity: A comparison. Med. Biol. Eng. Comput. 2009, 47, 883–892. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V. Alginate/chitosan microparticles for tamoxifen delivery to the lymphatic system. Int. J. Pharm. 2009, 367, 127–132. [Google Scholar] [CrossRef]
- Chen, C.C.; Fang, C.L.; Al-Suwayeh, S.A.; Leu, Y.L.; Fang, J.Y. Transdermal delivery of selegiline from alginate-Pluronic composite thermogels. Int. J. Pharm. 2011, 415, 119–128. [Google Scholar] [CrossRef]
- Balaure, P.C.; Andronescu, E.; Grumezescu, A.M.; Ficai, A.; Huang, K.S.; Yang, C.H.; Chifiriuc, C.M.; Lin, Y.S. Fabrication, characterization and in vitro profile based interaction with eukaryotic and prokaryotic cells of alginate-chitosan-silica biocomposite. Int. J. Pharm. 2013, 441, 555–561. [Google Scholar] [CrossRef]
- Barbetta, A.; Barigelli, E.; Dentini, M. Porous alginate hydrogels: Synthetic methods for tailoring the porous texture. Biomacromolecules 2009, 10, 2328–2337. [Google Scholar] [CrossRef]
- Wan, J.; Bick, A.; Sullivan, M.; Stone, H.A. Controllable microfluidic production of microbubbles in water-in-oil emulsions and the formation of porous microparticles. Adv. Mater. 2008, 20, 3314–3318. [Google Scholar] [CrossRef]
- Cavalieri, F.; Zhou, M.; Tortora, M.; Lucilla, B.; Ashokkumar, M. Methods of preparation of multifunctional microbubbles and their in vitro/in vivo assessment of stability, functional and structural properties. Curr. Pharm. Des. 2012, 18, 2135–2151. [Google Scholar] [CrossRef]
- Soetanto, K.; Chan, M.; Okujima, M. Effect of calcium chloride on sodium alginate microbubbles as ultrasound contrast agent. Jpn. J. Appl. Phys. 1996, 35, 3152–3155. [Google Scholar] [CrossRef]
- Sridhar-Keralapura, M.; Thirumalai, S.; Mobed-Miremadi, M. Structural changes and imaging signatures of acoustically sensitive microcapsules under ultrasound. Ultrasonics 2013, 53, 1044–1057. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Lin, Y.S.; Wang, C.Y.; Lu, K.; Chang, Y.F.; Wang, Y.L. Electrostatic droplets assisted synthesis of alginate microcapsules. Drug Deliv. Transl. Res. 2011, 1, 289–298. [Google Scholar] [CrossRef]
- Huang, K.S.; Lin, Y.S.; Yang, C.H.; Tsai, C.W.; Hsu, M.Y. In situ synthesis of twin monodispersed alginate microparticles. Soft Matter 2011, 7, 6713–6718. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, C.H.; Lin, Y.S.; Chen, C.H.; Huang, K.S. Anti-inflammatory effect with high intensity focused ultrasound-mediated pulsatile delivery of diclofenac. Biomaterials 2012, 33, 1547–1553. [Google Scholar] [CrossRef]
- Lin, Y.S.; Yang, C.H.; Hsu, Y.Y.; Hsieh, C.L. Microfluidic synthesis of tail-shaped alginate microparticles using slow sedimentation. Electrophoresis 2013, 34, 425–431. [Google Scholar] [CrossRef]
- Gong, X.; Wen, W.; Sheng, P. Microfluidic fabrication of porous polymer microspheres: Dual reactions in single droplets. Langmuir 2009, 25, 7072–7077. [Google Scholar] [CrossRef]
- Firsova, T.P.; Sokol, V.I.; Bakulina, V.M.; Stasevich, N.N. Reaction of sodium bicarbonate with hydrogen peroxide and some properties of the compound Na2CO3 1.5 H2O2. Russ. Chem. Bull. 1968, 17, 1850–1853. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, K.-S.; Lin, Y.-S.; Chang, W.-R.; Wang, Y.-L.; Yang, C.-H. A Facile Fabrication of Alginate Microbubbles Using a Gas Foaming Reaction. Molecules 2013, 18, 9594-9602. https://doi.org/10.3390/molecules18089594
Huang K-S, Lin Y-S, Chang W-R, Wang Y-L, Yang C-H. A Facile Fabrication of Alginate Microbubbles Using a Gas Foaming Reaction. Molecules. 2013; 18(8):9594-9602. https://doi.org/10.3390/molecules18089594
Chicago/Turabian StyleHuang, Keng-Shiang, Yung-Sheng Lin, Wan-Ru Chang, Yi-Ling Wang, and Chih-Hui Yang. 2013. "A Facile Fabrication of Alginate Microbubbles Using a Gas Foaming Reaction" Molecules 18, no. 8: 9594-9602. https://doi.org/10.3390/molecules18089594



