Enzymatic Synthesis of Extremely Pure Triacylglycerols Enriched in Conjugated Linoleic Acids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipase-Catalyzed Synthesis of CLA-Enriched TAG
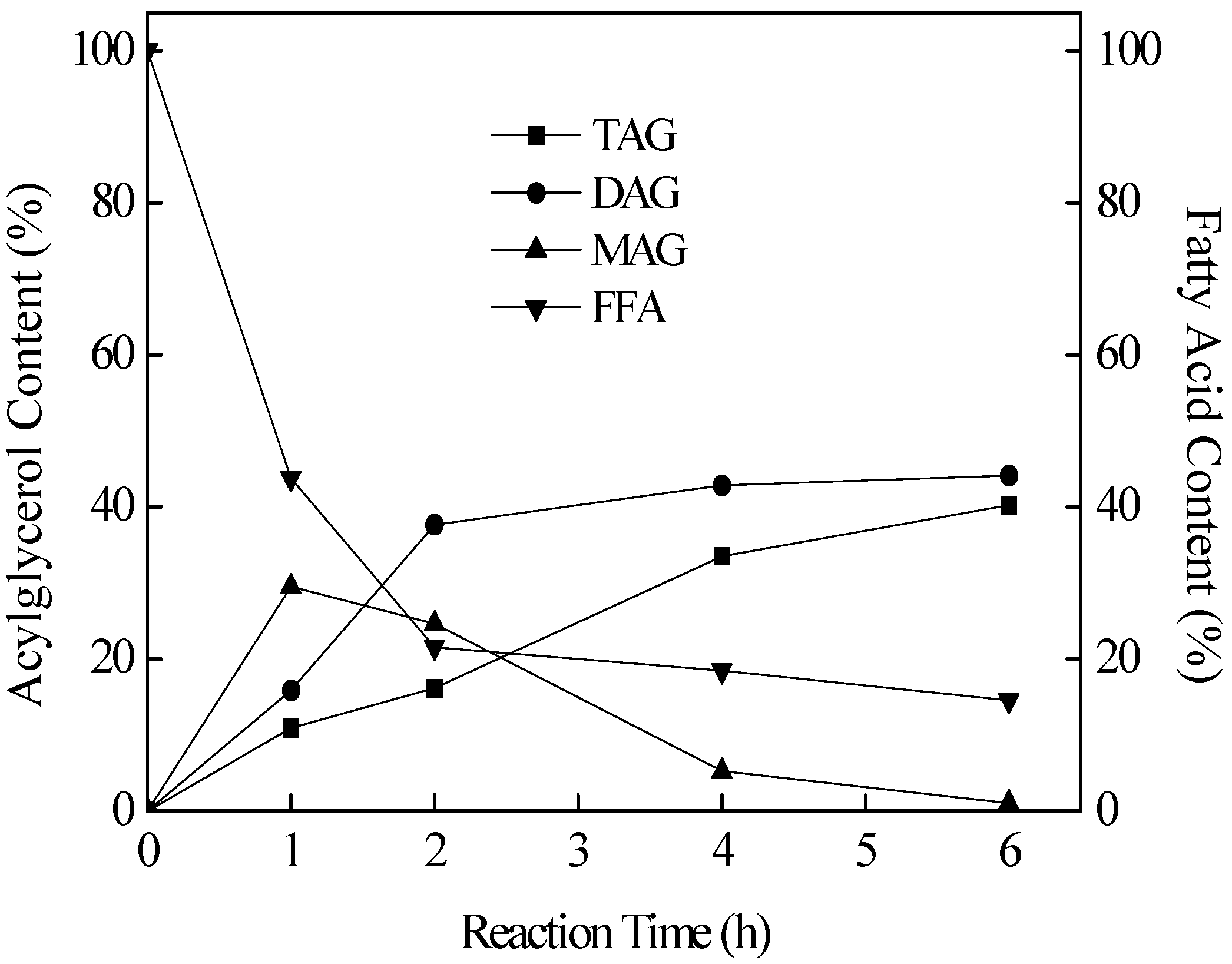
2.2. Lipase SMG1-Catalyzed Hydrolysis of Lower Glycerides in TAG Products
2.2.1. Model Analysis to Estimate the Coefficients

| Trials | X1 | X2 | X3 | Purity of TAG (%) | |
|---|---|---|---|---|---|
| Observed | Predicted | ||||
| 1 | −1(30) | −1(5) | −1(20) | 91.5 ± 0.14 | 91.47 |
| 2 | 1(90) | −1(5) | −1(20) | 91.3 ± 0.17 | 91.37 |
| 3 | −1(30) | 1(6) | −1(20) | 92.3 ± 0.11 | 92.31 |
| 4 | 1(90) | 1(6) | −1(20) | 98.3 ± 0.13 | 98.21 |
| 5 | −1(30) | −1(5) | 1(30) | 86.3 ± 0.10 | 86.37 |
| 6 | 1(90) | −1(5) | 1(30) | 91.9 ± 0.16 | 91.87 |
| 7 | −1(30) | 1(6) | 1(30) | 82.5 ± 0.17 | 82.41 |
| 8 | 1(90) | 1(6) | 1(30) | 93.9 ± 0.12 | 93.92 |
| 9 | −1.68(9.5) | 0(5.5) | 0(25) | 89.1 ± 0.09 | 89.12 |
| 10 | 1.68(110.4) | 0(5.5) | 0(25) | 98.7 ± 0.11 | 98.71 |
| 11 | 0(60) | −1.68(4.6) | 0(25) | 84.8 ± 0.14 | 84.75 |
| 12 | 0(60) | 1.68(6.3) | 0(25) | 87.1 ± 0.14 | 87.18 |
| 13 | 0(60) | 0(5.5) | −1.68(16.6) | 98.5 ± 0.11 | 98.51 |
| 14 | 0(60) | 0(5.5) | 1.68(33.4) | 90.6 ± 0.13 | 90.61 |
| 15 | 0(60) | 0(5.5) | 0(25) | 99.5 ± 0.08 | 99.53 |
| 16 | 0(60) | 0(5.5) | 0(25) | 99.5 ± 0.11 | 99.53 |
| 17 | 0(60) | 0(5.5) | 0(25) | 99.7 ± 0.12 | 99.53 |
| 18 | 0(60) | 0(5.5) | 0(25) | 99.4 ± 0.10 | 99.53 |
| 19 | 0(60) | 0(5.5) | 0(25) | 99.5 ± 0.13 | 99.53 |
| 20 | 0(60) | 0(5.5) | 0(25) | 99.6 ± 0.12 | 99.53 |
2.2.2. Main Effects and Interaction between Parameters
| Source | Degree of freedom | Mean square | F value | Prob > F |
|---|---|---|---|---|
| Model | 9 | 69.21 | 7728.11 | <0.0001 |
| X1 | 1 | 111.06 | 12400.74 | <0.0001 |
| X2 | 1 | 7.13 | 796.18 | <0.0001 |
| X3 | 1 | 75.38 | 8417.34 | <0.0001 |
| X1X2 | 1 | 18.00 | 2009.85 | <0.0001 |
| X1X3 | 1 | 15.68 | 1750.80 | <0.0001 |
| X2X3 | 1 | 11.52 | 1286.30 | <0.0001 |
| X12 | 1 | 56.89 | 6352.52 | <0.0001 |
| X22 | 1 | 331.71 | 37038.25 | <0.0001 |
| X32 | 1 | 44.49 | 4968.01 | <0.0001 |
| Residual | 10 | 8.956E-003 | ||
| Lack of Fit | 5 | 7.245E-003 | 0.68 | 0.6592 |
| Pure Error | 5 | 0.011 |
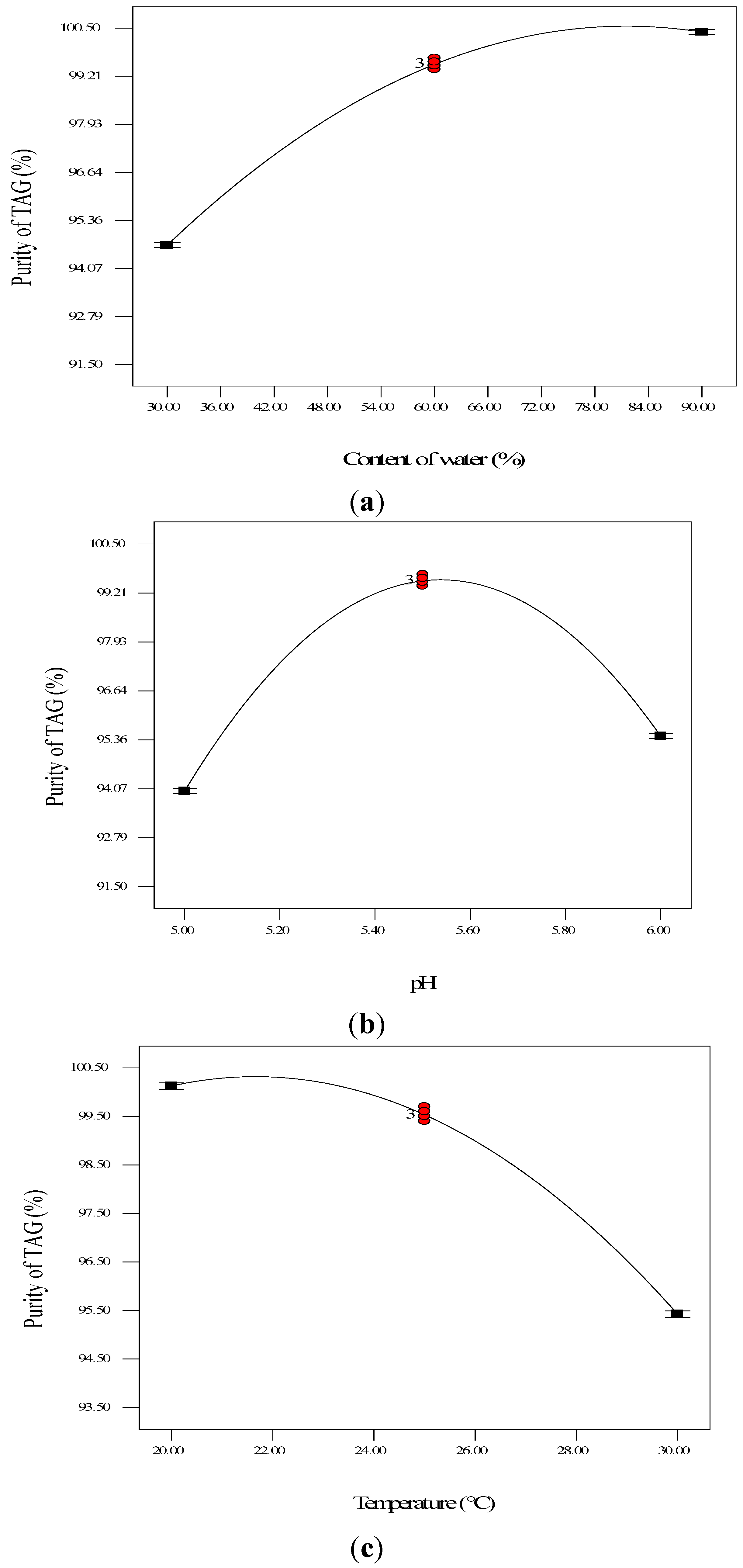
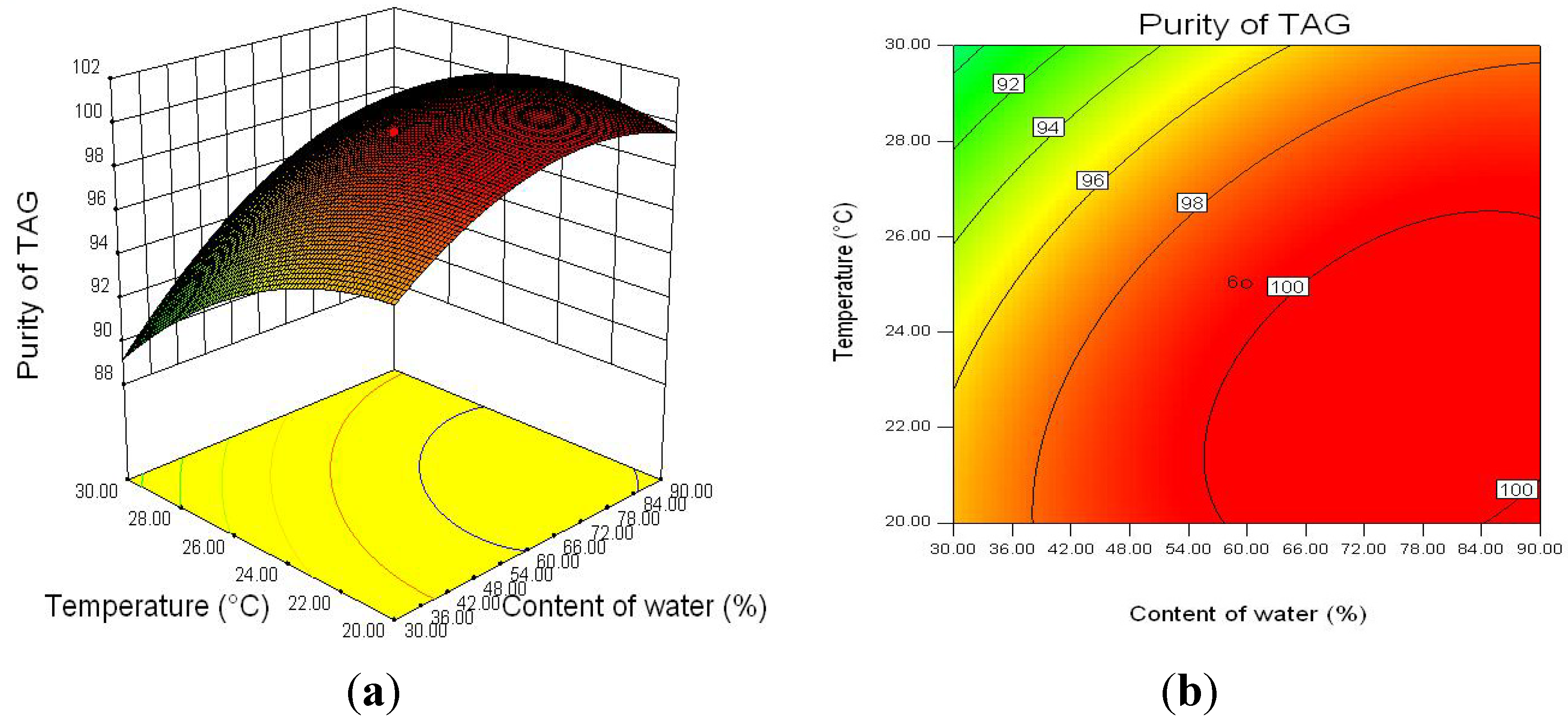
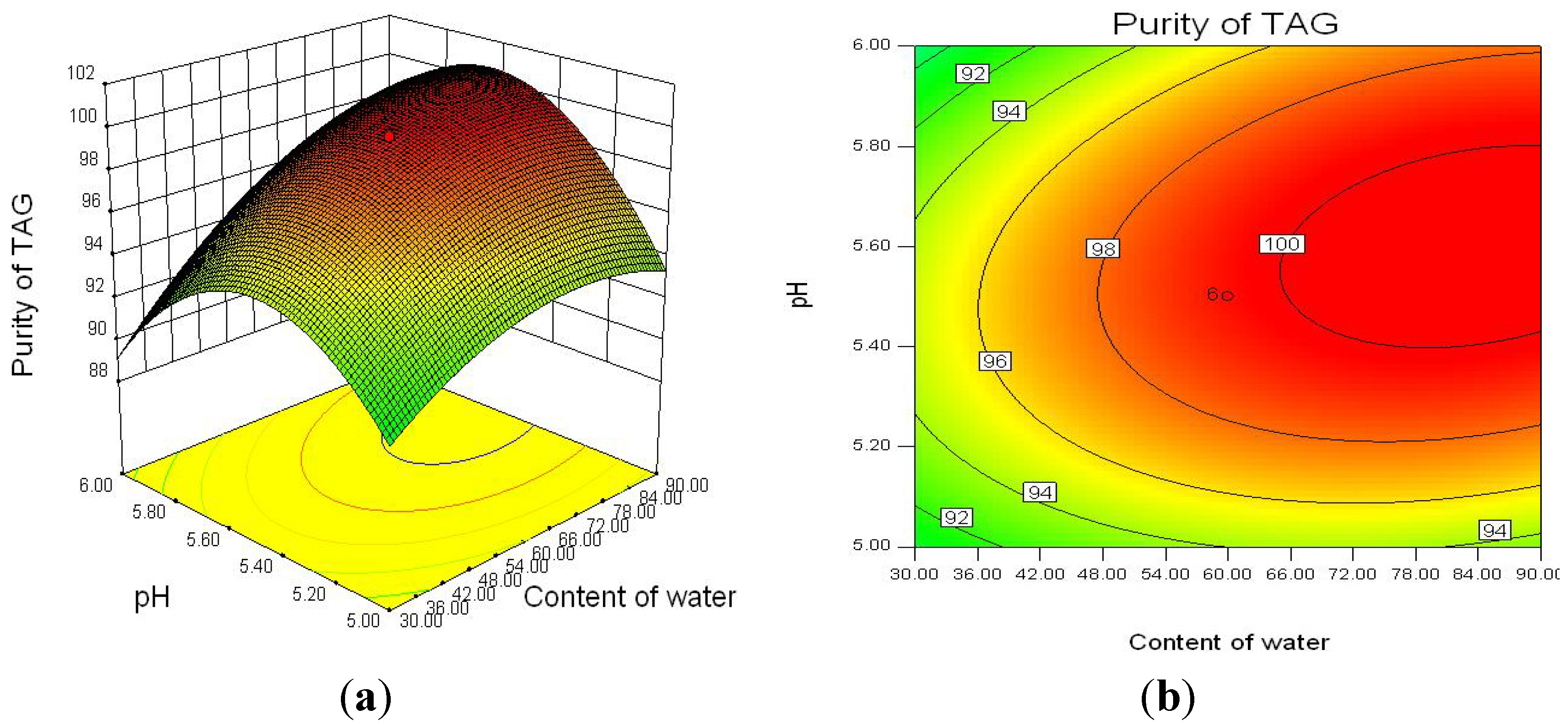
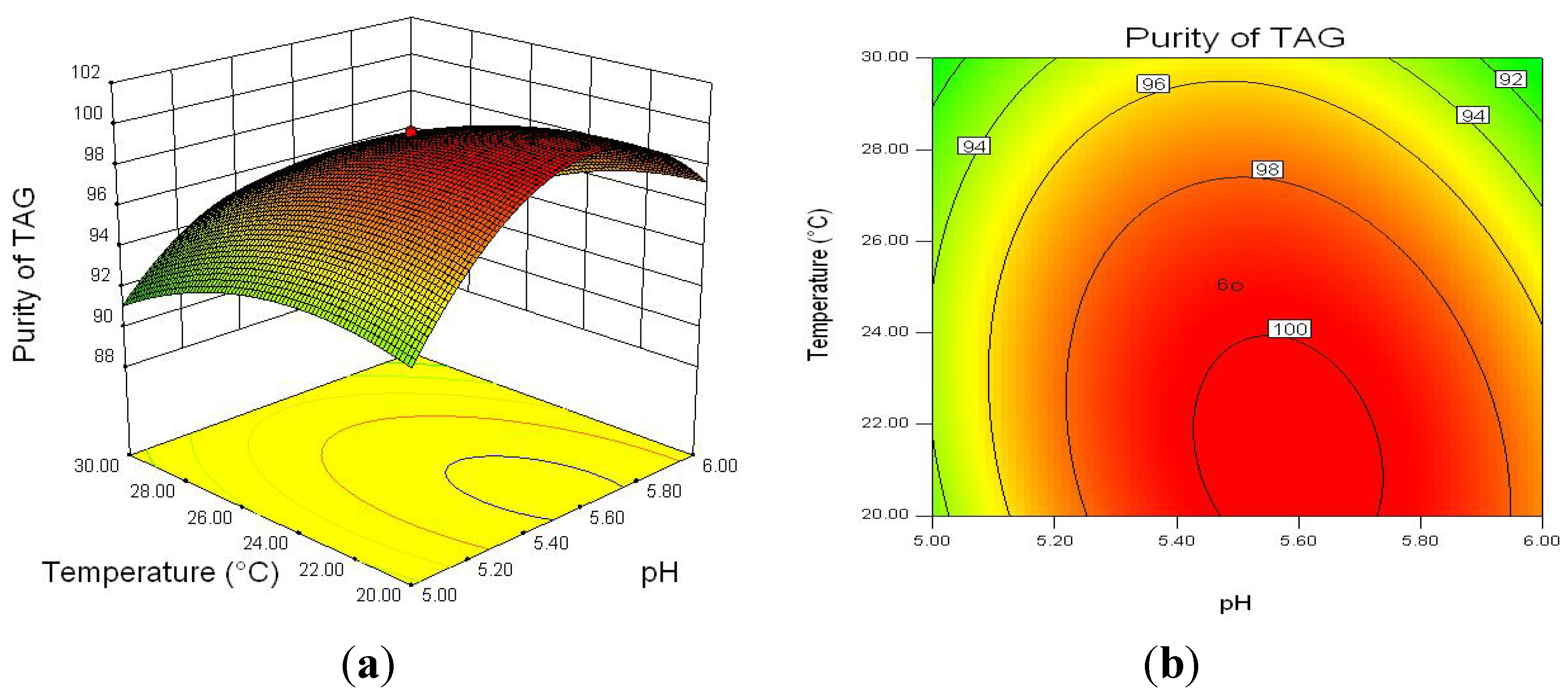
2.2.3. Optimization of Enzymatic Hydrolysis Parameters for TAG Purity
2.3. Purification of TAG by Molecular Distillation
| CLA | CLA-enriched TAG | |
|---|---|---|
| Acylglycerol Profile (%) | ||
| TAG | 0 | 99.8 |
| DAG | 0 | 0.2 |
| MAG | 0 | 0 |
| FA | 100 | 0 |
| Fatty Acid Compositions (%) | ||
| C16:0 | 4.74 | 4.95 |
| C18:0 | 2.22 | 2.32 |
| C18:1 | 11.47 | 11.56 |
| C18:2 | 0.95 | 0.97 |
| Total CLA | 80.62 | 80.22 |
| c9,t11-CLA | 33.95 | 31.78 |
| t10,c12-CLA | 43.59 | 44.92 |
| Other CLA | 3.08 | 3.52 |
3. Experimental
3.1. Materials
3.2. Preparation of CLA Glycerides
3.3. Experimental Design

3.4. Lipase SMG1-Catalyzed Hydrolysis of Lower Glycerides
3.5. Purification of the TAG by Molecular Distillation
3.6. HPLC Analysis of the Products

3.7. Analysis of Fatty Acid Compositions of TAGs
3.8. Other Analyses
3.9. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhong, X.F.; Luo, T.; Huang, G.D.; Deng, Z.Y.; Lei, L. Equimolar mixture of c9,t11 and t9,t11 CLA inhibits the growth and induces apoptosis in Caco-2 cells. Eur. J. Lipid. Sci. Technol. 2012, 114, 479–485. [Google Scholar] [CrossRef]
- Whigham, L.D.; Cook, M.E.; Atkinson, R.L. Conjugated linoleic acid: Implications for human health. Pharmacol. Res. 2000, 42, 503–510. [Google Scholar] [CrossRef]
- Belury, M.A. Inhibition of carcinogenesis by conjugated linoleic acid: Potential mechanisms of action. J. Nutr. 2002, 132, 2995–2998. [Google Scholar]
- Oraldi, M.; Maggiora, M.; Paiuzzi, E.; Canuto, R.A.; Muzio, G. CLA Reduces Inflammatory Mediators from A427 Human Lung Cancer Cells and A427 Conditioned Medium Promotes Differentiation of C2C12 Murine Muscle Cells. Lipids 2013, 48, 29–38. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Jones, E.L.; Grimble, R.F.; Williams, C.M.; Yagoob, P.; Calder, P.C. Opposing effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am. J. Clin. Nutr. 2004, 80, 614–620. [Google Scholar]
- Miner, J.L.; Cederberg, C.A.; Nielsen, M.K.; Chen, X.; Baile, C.A. Conjugated Linoleic Acid (CLA), Body Fat, and Apoptosis. Obes. Res. 2012, 9, 129–134. [Google Scholar]
- Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J. Int. Med. Res. 2001, 29, 392–396. [Google Scholar] [CrossRef]
- Terpstra, A.; Javadi, M.; Beynen, A.; Kocsis, S.; Lankhorst, A.E.; Lemmens, A.G.; Mohede, I.C. Dietary conjugated linoleic acids as free fatty acids and triacylglycerols similarly affect body composition and energy balance in mice. J. Nutr. 2003, 133, 3181–3186. [Google Scholar]
- Fernie, C.E.; Dupont, I.E.; Scruel, O.; Carpentier, Y.A.; Sebedio, J.L.; Scrimgeour, C.M. Relative absorption of conjugated linoleic acid as triacylglycerol, free fatty acid and ethyl ester in a functional food matrix. Eur. J. Lipid. Sci. Tech. 2004, 106, 347–354. [Google Scholar] [CrossRef]
- Stewart, J.E.; Feinle-Bisset, C.; Keast, R.S. Fatty acid detection during food consumption and digestion: Associations with ingestive behavior and obesity. Prog. Lipid Res. 2011, 50, 225–233. [Google Scholar] [CrossRef]
- Medina, A.R.; Cerdán, L.E.; Giménez, A.G.; Páez, B.C.; González, M.J.I.; Grima, E.M. Lipase-catalyed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. J. Biotechnol. 1999, 70, 379–391. [Google Scholar] [CrossRef]
- Torres, C.F.; Garcia, H.S.; Ries, J.J.; Hill, C.G., Jr. Esterification of glycerol with conjugated linoleic acid and long-chain fatty acids from fish oil. J. Am. Oil Chem. Soc. 2001, 78, 1093–1098. [Google Scholar] [CrossRef]
- Busch, S.; Horlacher, P.; Both, S.; Westfechtel, A.; Schörken, U. Green synthesis routes toward triglycerides of conjugated linoleic acid. Eur. J. Lipid. Sci. Tech. 2011, 113, 92–99. [Google Scholar] [CrossRef]
- Wang, Y.H.; Jing, C.F.; Yang, B.; Mainda, G.; Dong, M.L.; Xu, A.L. Production of a new sea anemone neurotoxin by recombinant Escherichia coli: Optimization of culture conditions using response surface methodology. Process. Biochem. 2005, 40, 2721–2728. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.M.; Ou, S.Y.; Xie, L.Y.; Tang, S.Z. Preparation of a diacylglycerol-enriched soybean oil by phosphalipase A1 catalyzed hydrolysis. J. Mol. Catal. B Enzy. 2009, 56, 165–172. [Google Scholar] [CrossRef]
- Wang, W.F.; Li, T.; Qin, X.L.; Ning, Z.X.; Yang, B.; Wang, Y.H. Production of lipase SMG1 and its application in synthesizing diacylglyecrol. J. Mol. Catal. B Enzy. 2012, 77, 87–91. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, X.F.; Liang, Y.C.; Yang, B.; Zhang, S.J. Enzymatic fractionation of conjugated linoleic acid isomers by selective esterification. J. Mol. Catal. B Enzy. 2007, 46, 20–25. [Google Scholar] [CrossRef]
- Hong, S.I.; Kim, Y.; Yoon, S.W.; Cho, S.Y.; Kim, I.H. Synthesis of CLA-enriched TAG byCandida antarcticalipase under vacuum. Eur. J. Lipid Sci. Tech. 2012, 114, 1044–1051. [Google Scholar] [CrossRef]
- European Committee for Standardization, Animal and vegetable fats and oils—Gas chromatography of fatty acid methyl esters - Part 2: Preparation of methyl esters of fatty acids. 2011; ISO Method 12966–2.
- Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1993; Methods Ca–5a.
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for detecmination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar]
- Sample Availability: Samples of the compounds CLA-enriched TAG is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cao, Y.; Wang, W.; Xu, Y.; Yang, B.; Wang, Y. Enzymatic Synthesis of Extremely Pure Triacylglycerols Enriched in Conjugated Linoleic Acids. Molecules 2013, 18, 9704-9716. https://doi.org/10.3390/molecules18089704
Cao Y, Wang W, Xu Y, Yang B, Wang Y. Enzymatic Synthesis of Extremely Pure Triacylglycerols Enriched in Conjugated Linoleic Acids. Molecules. 2013; 18(8):9704-9716. https://doi.org/10.3390/molecules18089704
Chicago/Turabian StyleCao, Yu, Weifei Wang, Yang Xu, Bo Yang, and Yonghua Wang. 2013. "Enzymatic Synthesis of Extremely Pure Triacylglycerols Enriched in Conjugated Linoleic Acids" Molecules 18, no. 8: 9704-9716. https://doi.org/10.3390/molecules18089704




