NMR Study on the Interaction of Trehalose with Lactose and Its Effect on the Hydrogen Bond Interaction in Lactose
Abstract
:1. Introduction
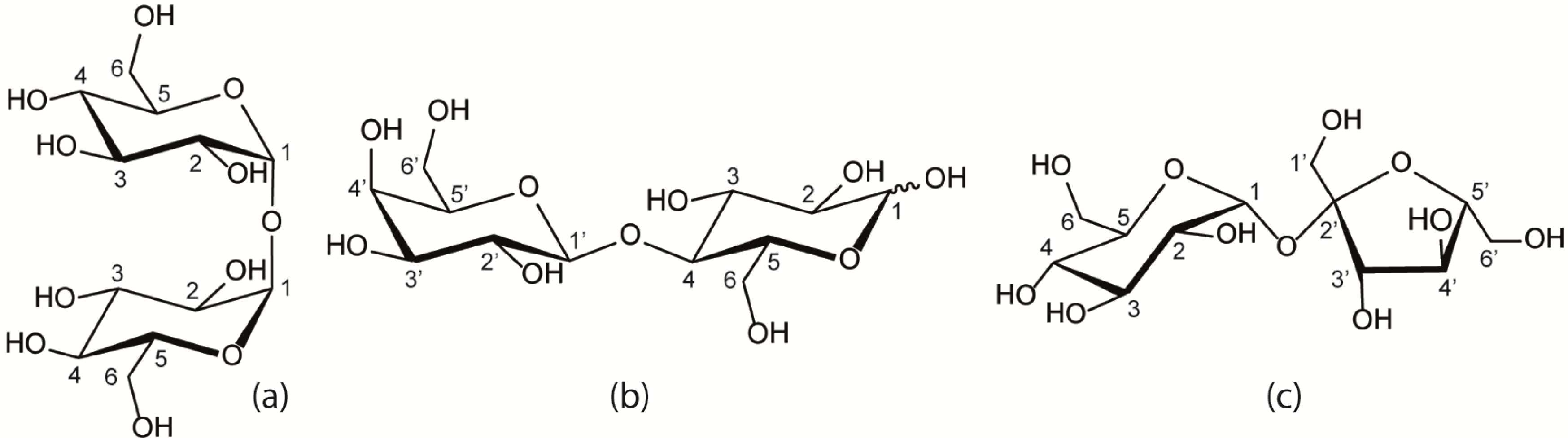
2. Results and Discussion
2.1. Diffusion in Binary and Ternary Systems
2.1.1. Binary Water/Trehalose and Water/Lactose Systems
 Lac,
Lac,  D2O. Lac 253 mg/mL:
D2O. Lac 253 mg/mL:  Lac,
Lac,  D2O. Tre 34 mg/mL:
D2O. Tre 34 mg/mL:  Tre,
Tre,  D2O. Tre 258 mg/mL:
D2O. Tre 258 mg/mL:  Tre,
Tre,  D2O.
D2O.
 Lac,
Lac,  D2O. Lac 253 mg/mL:
D2O. Lac 253 mg/mL:  Lac,
Lac,  D2O. Tre 34 mg/mL:
D2O. Tre 34 mg/mL:  Tre,
Tre,  D2O. Tre 258 mg/mL:
D2O. Tre 258 mg/mL:  Tre,
Tre,  D2O.
D2O.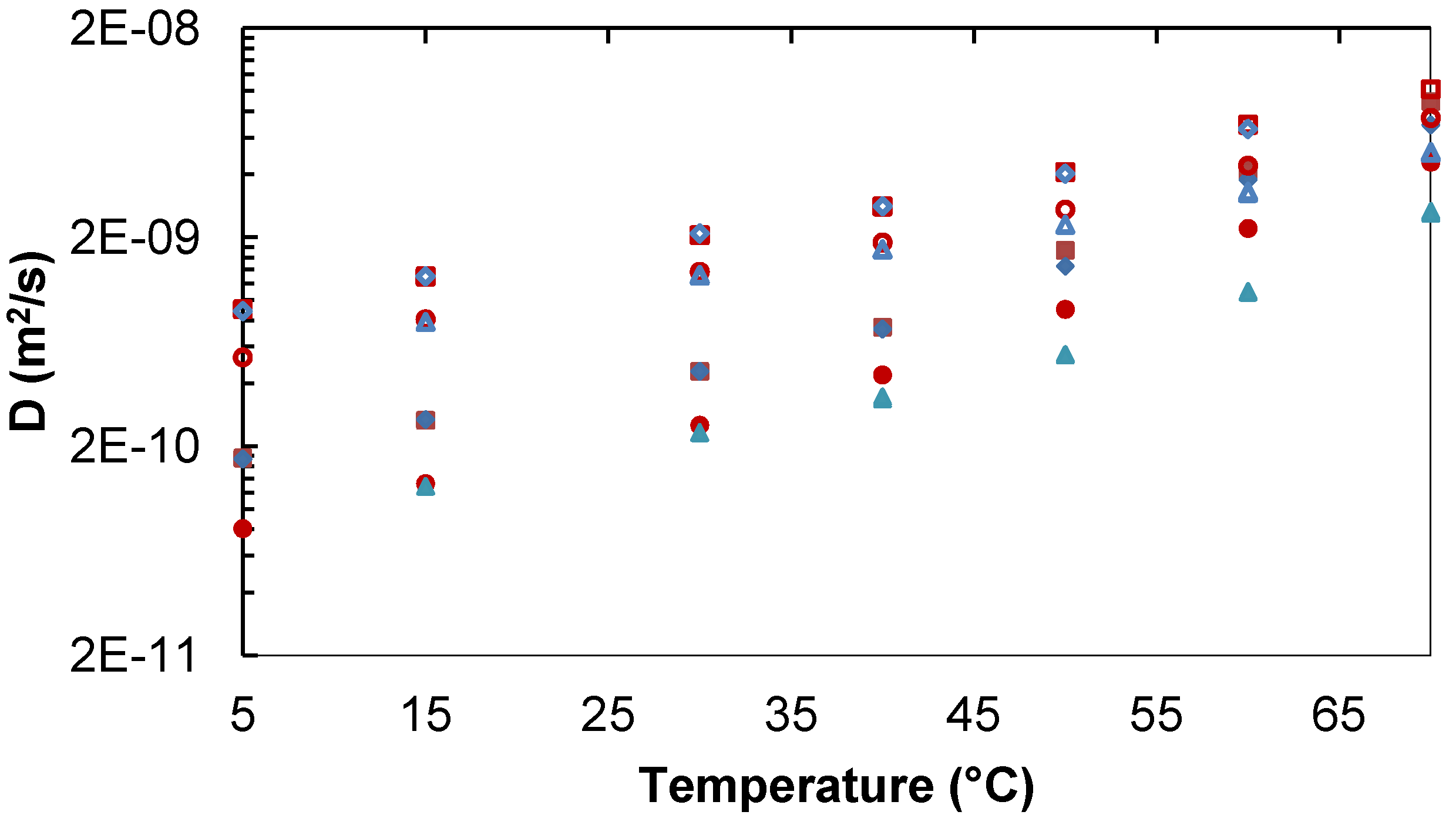
2.1.2. Ternary Water/Trehalose/Lactose Systems
 Tre,
Tre,  Lac,
Lac,  D2O. Tre/Lac 170/170 mg/mL:
D2O. Tre/Lac 170/170 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O. Tre/Lac 254/254 mg/mL:
D2O. Tre/Lac 254/254 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O.
D2O.
 Tre,
Tre,  Lac,
Lac,  D2O. Tre/Lac 170/170 mg/mL:
D2O. Tre/Lac 170/170 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O. Tre/Lac 254/254 mg/mL:
D2O. Tre/Lac 254/254 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O.
D2O.
2.1.3. Comparison of Trehalose/Lactose/Water and Trehalose/Glucose/Water systems
 Tre,
Tre,  Lac,
Lac,  D2O. Tre/Glc 35/20 mg/mL: ♦Tre,
D2O. Tre/Glc 35/20 mg/mL: ♦Tre,  Glc,
Glc,  D2O. Tre/Lac 780/63 mg/mL:
D2O. Tre/Lac 780/63 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O. Tre/Glc 785/67 mg/mL: ●Tre,
D2O. Tre/Glc 785/67 mg/mL: ●Tre,  Glc,
Glc,  D2O.
D2O.
 Tre,
Tre,  Lac,
Lac,  D2O. Tre/Glc 35/20 mg/mL: ♦Tre,
D2O. Tre/Glc 35/20 mg/mL: ♦Tre,  Glc,
Glc,  D2O. Tre/Lac 780/63 mg/mL:
D2O. Tre/Lac 780/63 mg/mL:  Tre,
Tre,  Lac,
Lac,  D2O. Tre/Glc 785/67 mg/mL: ●Tre,
D2O. Tre/Glc 785/67 mg/mL: ●Tre,  Glc,
Glc,  D2O.
D2O.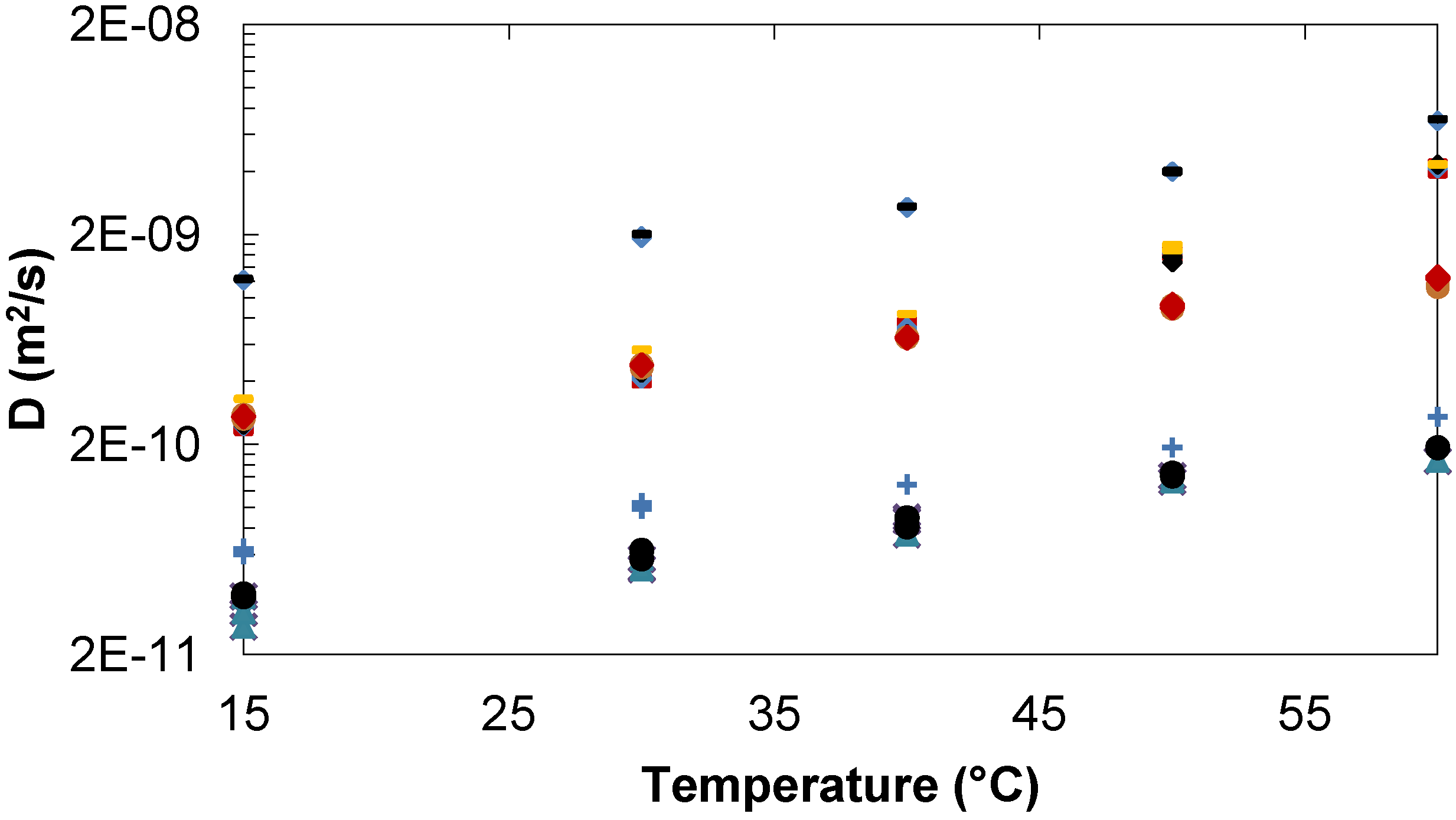
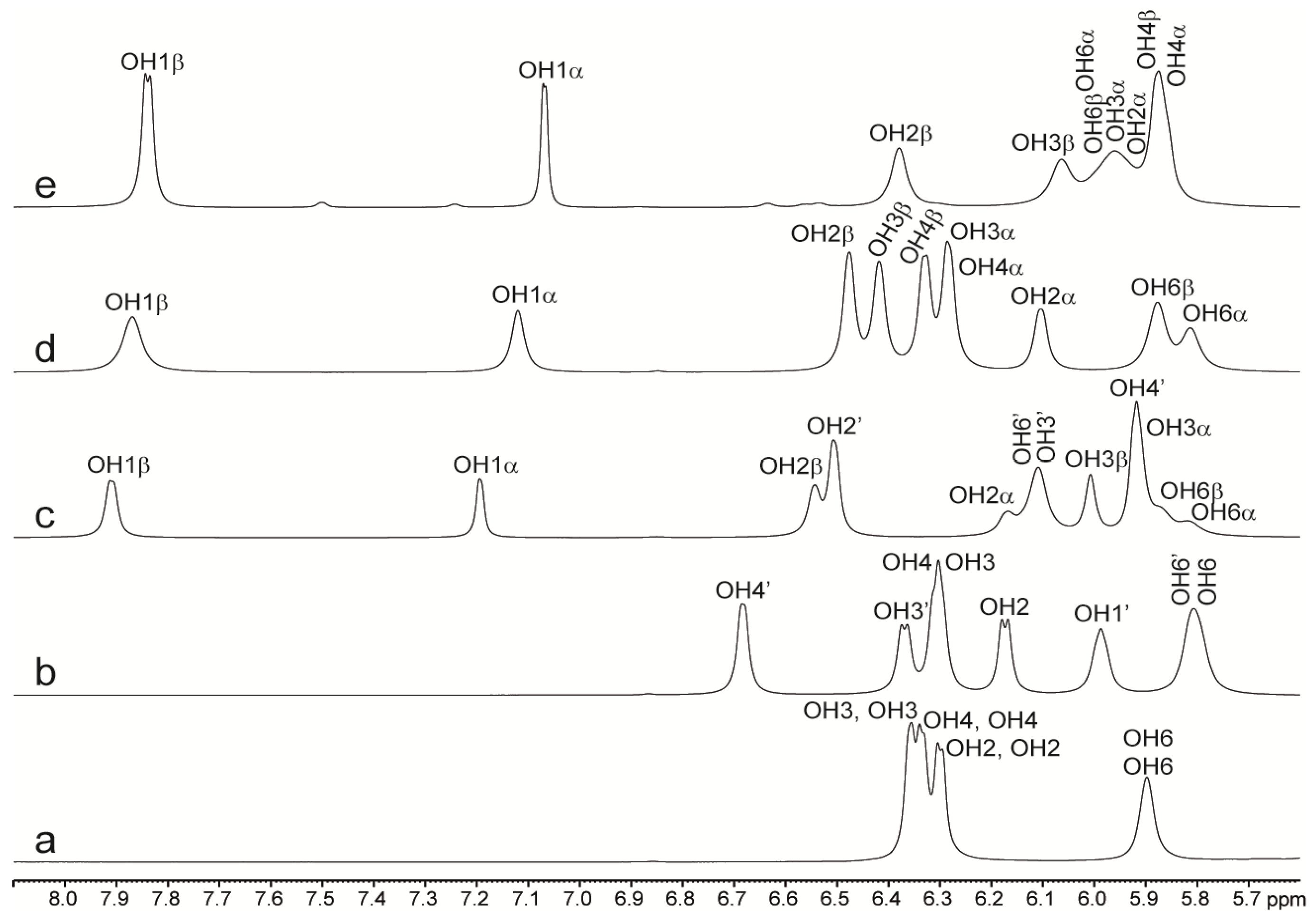
2.2. NMR of Hydroxy Protons
2.2.1. Binary Systems
| Lactose | 35 mg/mL | 203 mg/mL | ||
| 3JCH, OH/dδ/dT | 3JCH, OH/dδ/dT | |||
| GlcOH1α | 4.2/−8.7 | 4.4/−8.4 | ||
| GlcOH1β | 5.5/−9.0 | 6.5/−9.8 | ||
| GlcOH2α | 5.9/−11.4 | ----/−11.3 | ||
| GlcOH2β | 4.9/−11.8 | 4.8/−11.6 | ||
| GlcOH3α | 3.5/−10.4 | ----/−10.2 | ||
| GlcOH3β | 3.5/−10.8 | 3.3/−10.8 | ||
| GlcOH6α | ----/−11.1 | ----/−10.7 | ||
| GlcOH6β | ----/−11.7 | ----/−10.9 | ||
| GalOH2 | 5.4/−10.7 | 5.4/−10.6 | ||
| GalOH3 | 5.9/−10.5 | ----/−10.3 | ||
| GalOH4 | 5.1/−10.9 | ----/−10.8 | ||
| GalOH6 | 5.6/−12.7 | ----/−12.3 | ||
| Trehalose | 35 mg/mL | 206 mg/mL | 338 mg/mL | |
| 3JCH, OH/dδ/dT | 3JCH, OH/dδ/dT | 3JCH, OH/dδ/dT | ||
| OH2 | 6.9/−11.0 | 7.0/−10.8 | 7.0/−10.6 | |
| OH3 | 5.2/−11.8 | 5.1/−11.6 | 5.0/−11.3 | |
| OH4 | 6.5/−10.5 | 6.5/−10.4 | 6.4/−10.1 | |
| OH6 | ----/−11.8 | ----/−11.4 | ----/−10.9 | |
| OH6 | ----/−11.8 | ----/−11.4 | ----/−10.9 | |
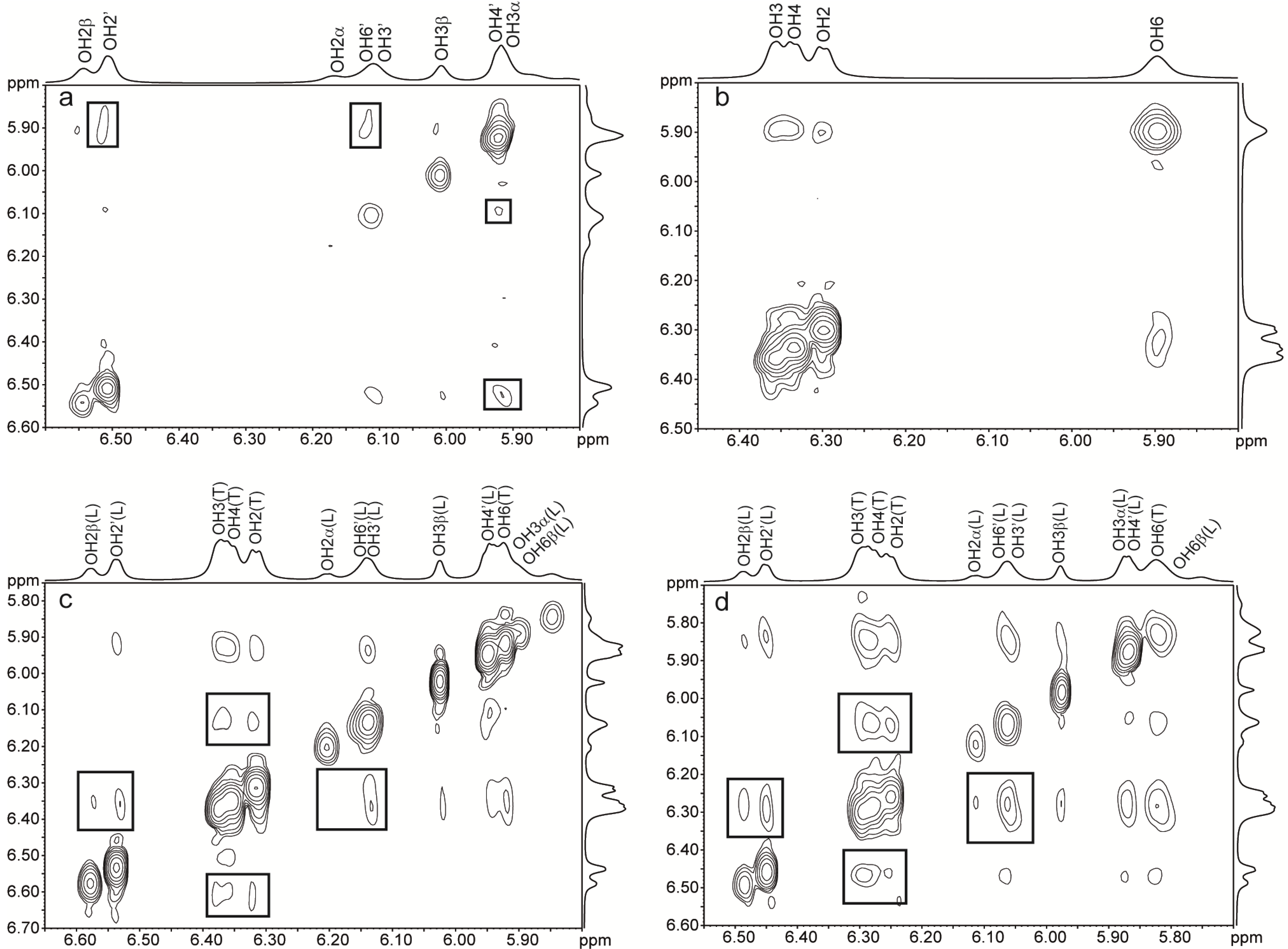
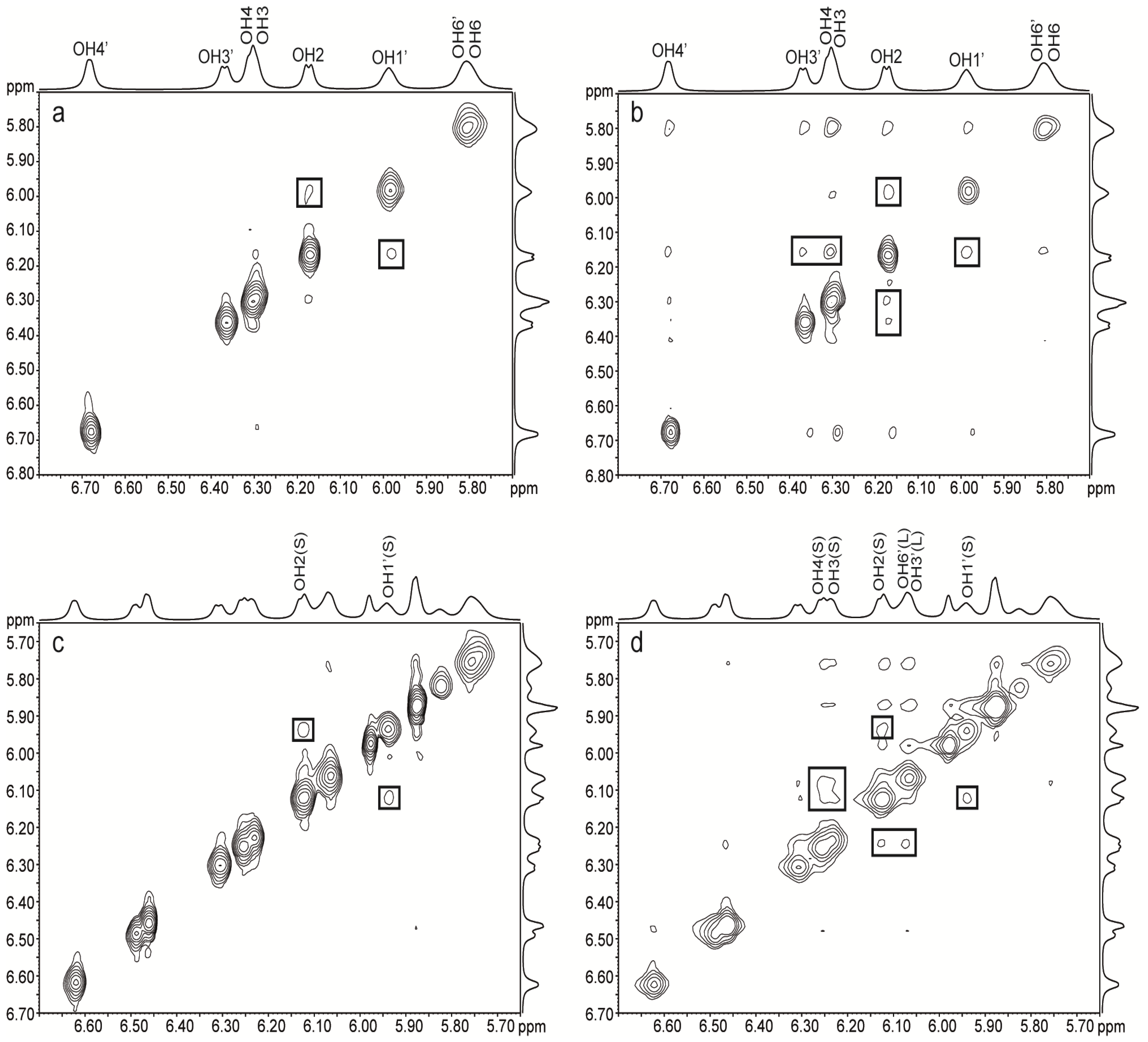
2.2.2. Ternary Systems
| Tre/Lac | 37 + 36 mg/mL | 256 + 256 mg/mL | 383 + 380 mg/mL | 593 + 54 mg/mL |
|---|---|---|---|---|
| Trehalose | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT |
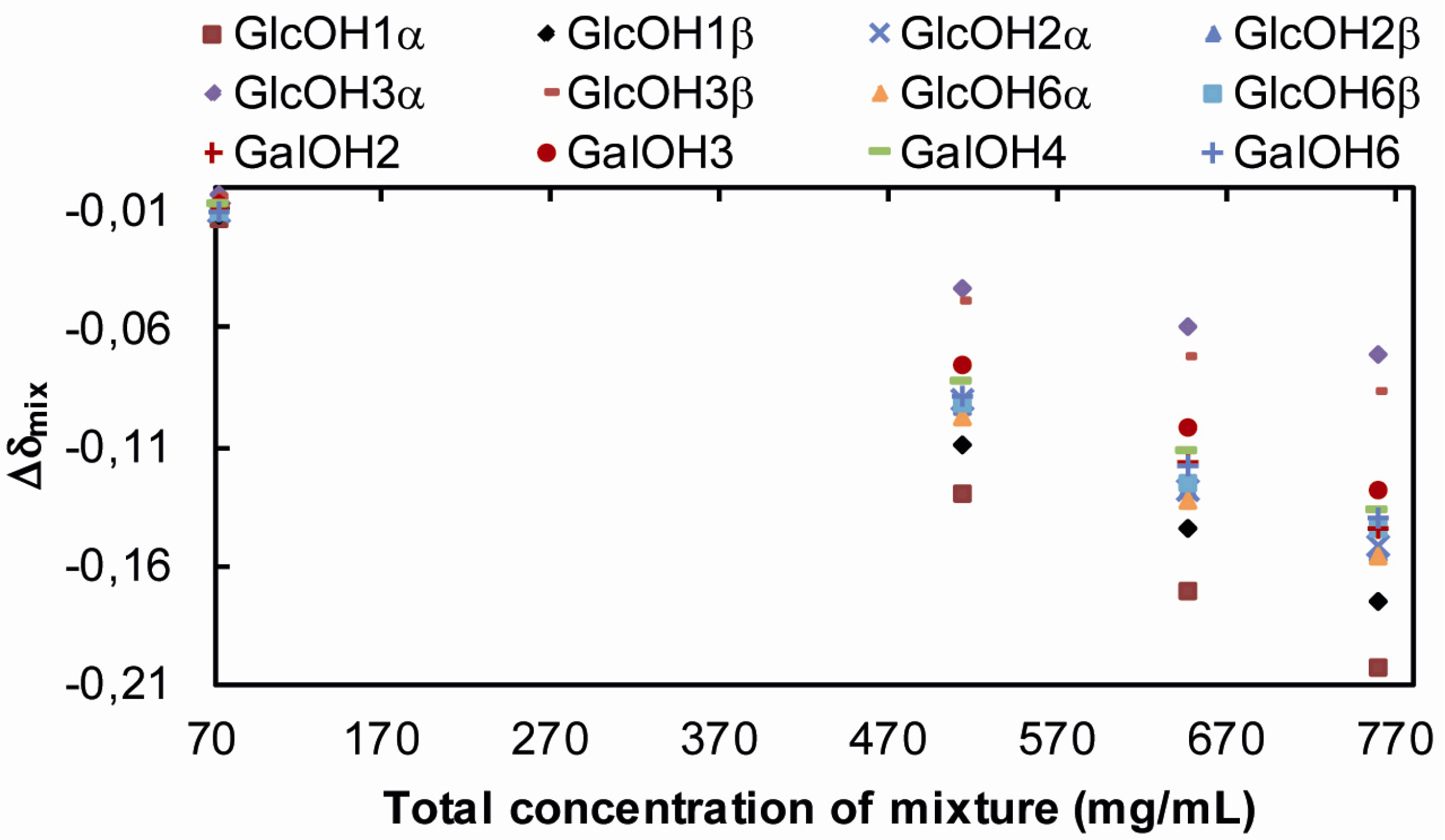
| OH2 | 7.0/−0.005/−11.0 | 6.8/−0.066/−10.3 | ----/−0.111/−10.4 | 6.7/−0.092/−10.4 |
| OH3 | 5.2/−0.006/−11.8 | 4.6/−0.077/−11.0 | ----/−0.132/−10.9 | 4.7/−0.106/−11.0 |
| OH4 | 6.6/−0.007/−10.4 | 6.4/−0.076/−9.8 | ----/−0.125/−10.0 | 6.3/−0.104/−10.0 |
| OH6 | ----/−0.011/−11.8 | ----/−0.104/−10.5 | ----/−0.167/−10.3 | ----/−0.144/−10.2 |
| OH6 | ----/−0.011/−11.8 | ----/−0.104/−10.5 | ----/−0.167/−10.3 | ----/−0.144/−10.2 |
| Tre/Lac | 37 + 36 mg/ml | 256 + 256 mg/ml | 383 + 380 mg/ml | 593 + 54 mg/ml |
|---|---|---|---|---|
| Lactose | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT |
| GlcOH1α | 4.1/−0.014/−8.6 | 3.7/−0.129/−7.6 | ----/−0.204/−7.4 | ----/−0.170/−7.8 |
| GlcOH1β | 6.4/−0.012/−9.1 | 5.9/−0.109/−8.1 | ----/−0.175/−8.6 | 5.9/−0.145/−9.0 |
| GlcOH2α | 6.1/−0.011/−11.3 | 5.7/−0.090/−10.5 | ----/−0.152/−10.5 | 5.7/−0.128/−10.4 |
| GlcOH2β | 4.9/−0.011/−11.8 | 4.6/−0.093/10.9 | ----/−0.155/−10.9 | 4.3/−0.128/−10.9 |
| GlcOH3α | ----/−0.003/−10.5 | ----/−0.043/−10.2 | ----/−0.072/−9.2 | ----/−0.060/−9.9 |
| GlcOH3β | 3.4/−0.005/−10.8 | 2.8/−0.049/−9.9 | ----/−0.088/−9.6 | ----/−0.072/−9.9 |
| GlcOH6α | ----/−0.011/−11.0 | ----/−0.097/−9.9 | ----/−0.156/−9.7 | ----/−0.133/−9.8 |
| GlcOH6β | ----/−0.011/−11.2 | ----/−0.091/−10.0 | ----/−0.144/−10.1 | ----/−0.125/−10.2 |
| GalOH2 | 5.4/−0.009/−10.6 | 5.1/−0.088/−9.9 | ----/−0.145/−10.0 | 4.8/−0.117/−10.1 |
| GalOH3 | 5.8/−0.008/−10.5 | ----/−0.075/−9.7 | ----/−0.128/−9.4 | ----/−0.101/−9.5 |
| GalOH4 | ----/−0.008/−10.9 | ----/−0.083/−10.1 | ----/−0.138/−10.0 | ----/−0.112/−10.1 |
| GalOH6 | ----/−0.011/−12.4 | ----/−0.089/−11.3 | ----/−0.140/−11.4 | ----/−0.118/−11.2 |
| Sucrose/Lactose | Glucose/Lactose | |
|---|---|---|
| Lactose | 178 + 181 mg/mL | 239 + 240 mg/mL |
| 3JCH, OH/Δδmix/dδ/dT | 3JCH, OH/Δδmix/dδ/dT | |
| GlcOH1α | 4.1/−0.112/−7.9 | 4.2/−0.137/−8.0 |
| GlcOH1β | 6.3/−0.096/−8.5 | 6.3/−0.120/−8.7 |
| GlcOH2α | ----/−0.090/−11.5 | ----/−0.091/−10.8 |
| GlcOH2β | 4.7/−0.091/−11.4 | 4.0/−0.095/−11.3 |
| GlcOH3α | ----/−0.045/−10.2 | ----/−0.038/−10.2 |
| GlcOH3β | 2.7/−0.050/−10.2 | ----/−0.053/−10.2 |
| GlcOH6α | ----/−0.086/−10.4 | ----/−0.100/−10.3 |
| GlcOH6β | ----/−0.078/−10.5 | ----/−0.098/−10.5 |
| GalOH2 | 5.2/−0.077/−10.3 | 5.1/−0.090/−10.3 |
| GalOH3 | 5.4/−0.075/−9.9 | ----/−0.075/−10.0 |
| GalOH4 | ----/−0.077/−10.4 | ----/−0.087/−10.4 |
| GalOH6 | ----/−0.071/−11.6 | ----/−0.095/−11.6 |
3. Experimental
3.1. Sample Preparation
3.2. NMR Experiments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hottiger, T.; Devirgilio, C.; Hall, M.N.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal-stability of proteins in-vitro. Eur. J. Biochem. 1994, 219, 187–193. [Google Scholar] [CrossRef]
- Iwahashi, H.; Obuchi, K.; Fujii, S.; Komatsu, Y. The correlative evidence suggesting that trehalose stabilizes membrane-structure in the yeast Saccharomyces cerevisiae. Cell. Mol. Biol. 1995, 41, 763–769. [Google Scholar]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar]
- Sampedro, J.G.; Cortes, P.; Munoz-Clares, R.A.; Fernandez, A.; Uribe, S. Thermal inactivation of the plasma membrane H+-ATPase from Kluyveromyces lactis. Protection by trehalose. Bba-Protein. Struct. Mol. Enzym. 2001, 1544, 64–73. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Guerra, G.; Pardo, J.P.; Uribe, S. Trehalose-mediated protection of the plasma membrane H+-ATPase from Kluyveromyces lactis during freeze-drying and rehydration. Cryobiology 1998, 37, 131–138. [Google Scholar] [CrossRef]
- Allison, S.D.; Chang, B.; Randolph, T.W.; Carpenter, J.F. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys. 1999, 365, 289–298. [Google Scholar] [CrossRef]
- Belton, P.S.; Gil, A.M. IR and Raman-spectroscopic studies of the interaction of trehalose with hen egg-white lysozyme. Biopolymers 1994, 34, 957–961. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256, 319–327. [Google Scholar] [CrossRef]
- Green, J.L.; Angell, C.A. Phase-relations and vitrification in saccharide-water solutions and the trehalose anomaly. J. Phys. Chem. 1989, 93, 2880–2882. [Google Scholar] [CrossRef]
- Branca, C.; Magazu, S.; Maisano, G.; Migliardo, P. α,α-Trehalose-water solutions. 3. Vibrational dynamics studies by inelastic light scattering. J. Phys. Chem. B 1999, 103, 1347–1353. [Google Scholar]
- Branca, C.; Magazu, S.; Maisano, G.; Migliardo, P. Anomalous cryoprotective effectiveness of trehalose: Raman scattering evidences. J. Chem. Phys. 1999, 111, 281–287. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Descamps, M.; Migliardo, F. How homogeneous are the trehalose, maltose, and sucrose water solutions? An insight from molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 11046–11057. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Guinet, Y.; Hedoux, A.; Paccou, L.; Prevost, D.; Descamps, M. Influence of homologous disaccharides on the hydrogen-bond network of water: Complementary Raman scattering experiments and molecular dynamics simulations. Carbohyd. Res. 2005, 340, 881–887. [Google Scholar] [CrossRef]
- Lerbret, A.; Affouard, F.; Bordat, P.; Hedoux, A.; Guinet, Y.; Descamps, M. Slowing down of water dynamics in disaccharide aqueous solutions. J. Non-Cryst. Solids. 2011, 357, 695–699. [Google Scholar] [CrossRef]
- Heyden, M.; Brundermann, E.; Heugen, U.; Niehues, G.; Leitner, D.M.; Havenith, M. Long-range influence of carbohydrates on the solvation dynamics of water-answers from terahertz absorption measurements and molecular modeling simulations. J. Am. Chem. Soc. 2008, 130, 5773–5779. [Google Scholar]
- Lee, S.L.; Debenedetti, P.G.; Errington, J.R. A computational study of hydration, solution structure, and dynamics in dilute carbohydrate solutions. J. Chem. Phys. 2005, 122, 204511–204520. [Google Scholar] [CrossRef]
- Heugen, U.; Schwaab, G.; Bruendermann, E.; Heyden, M.; Yu, X.; Leitner, D.M.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F.; Migliardo, P.; Romeo, G. α,α-Trehalose/water solutions. 5. Hydration and viscosity in dilute and semidilute disaccharide solutions. J. Phys. Chem. B 2001, 105, 10140–10145. [Google Scholar] [CrossRef]
- Verde, A.V.; Campen, R.K. Disaccharide topology induces slowdown in local water dynamics. J. Phys. Chem. B 2011, 115, 7069–7084. [Google Scholar] [CrossRef] [Green Version]
- Magno, A.; Gallo, P. Understanding the mechanisms of bioprotection: A comparative study of aqueous solutions of trehalose and maltose upon supercooling. J. Phys. Chem. Lett. 2011, 2, 977–982. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Hedoux, A.; Guinet, Y.; Descamps, M. How do trehalose, maltose, and sucrose influence some structural and dynamical properties of lysozyme? Insight from molecular dynamics simulations. J. Phys. Chem. B 2007, 111, 9410–9420. [Google Scholar] [CrossRef]
- Lupi, L.; Comez, L.; Paolantoni, M.; Perticaroli, S.; Sassi, P.; Morresi, A.; Ladanyi, B.M.; Fioretto, D. Hydration and aggregation in mono- and disaccharide aqueous solutions by gigahertz-to-terahertz light scattering and molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 14760–14767. [Google Scholar] [CrossRef]
- Conrad, P.B.; de Pablo, J.J. Computer simulation of the cryoprotectant disaccharide α,α-trehalose in aqueous solution. J. Phys. Chem. A 1999, 103, 4049–4055. [Google Scholar] [CrossRef]
- Corradini, D.; Strekalova, E.G.; Stanley, H.E.; Gallo, P. Microscopic mechanism of protein cryopreservation in an aqueous solution with trehalose. Sci. Rep. 2013, 3, 1–10. [Google Scholar]
- Engelsen, S.B.; Férez, S. Unique similarity of the asymmetric trehalose solid-state hydration and the diluted aqueous-solution hydration. J. Phys. Chem. B 2000, 104, 9301–9311. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Monteiro, C.; de Penhoat, C.H.; Perez, S. The diluted aqueous solvation of carbohydrates as inferred from molecular dynamics simulations and NMR spectroscopy. Biophys. Chem. 2001, 93, 103–127. [Google Scholar] [CrossRef]
- Lins, R.D.; Pereira, C.S.; Hünenberger, P.H. Trehalose–protein interaction in aqueous solution. Protein Struct. Funct. Genet. 2004, 55, 177–186. [Google Scholar] [CrossRef]
- Liu, Q.; Schmidt, R.K.; Teo, B.; Karplus, P.A.; Brady, J.W. Molecular dynamics studies of the hydration of α,α-trehalose. J. Am. Chem. Soc. 1997, 119, 7851–7862. [Google Scholar] [CrossRef]
- Nunes, S.C.C.; Lopes Jesus, A.J.; Joao Moreno, M.; Eusebio, M.E.S. Conformational preferences of α,α-trehalose in gas phase and aqueous solution. Carbohyd. Res. 2010, 345, 2048–2059. [Google Scholar] [CrossRef]
- Perić-Hassler, L.; Hansen, H.S.; Baron, R.; Hünenberger, P.H. Conformational properties of glucose-based disaccharides investigated using molecular dynamics simulations with local elevation umbrella sampling. Carbohyd. Res. 2010, 345, 1781–1801. [Google Scholar] [CrossRef]
- Sapir, L.; Harries, D. Linking trehalose self-association with binary aqueous solution equation of state. J. Phys. Chem. B 2011, 115, 624–634. [Google Scholar] [CrossRef]
- Batta, G.; Kover, K.E.; Gervay, J.; Hornyak, M.; Roberts, G.M. Temperature dependence of molecular conformation, dynamics, and chemical shift anisotropy of α,α-trehalose in D2O by NMR relaxation. J. Am. Chem. Soc. 1997, 119, 1336–1345. [Google Scholar]
- Bonanno, G.; Noto, R.; Fornili, S.L. Water interaction with α,α-trehalose: Molecular dynamics simulation. J. Chem. Soc. Faraday. Trans. 1998, 94, 2755–2762. [Google Scholar] [CrossRef]
- Iannilli, E.; Tettamanti, E.; Galantini, L.; Magazu, S. An integrated quasi-elastic light-scattering, pulse-gradient-spin-echo study on the transport properties of α,α-trehalose, sucrose, and maltose deuterium oxide solutions. J. Phys. Chem. B 2001, 105, 12143–12149. [Google Scholar] [CrossRef]
- Pagnotta, S.E.; Alegria, A.; Colmenero, J. Dynamical behavior of highly concentrated trehalose water solutions: A dielectric spectroscopy study. Phys. Chem. Chem. Phys. 2012, 14, 2991–2996. [Google Scholar] [CrossRef]
- Paolantoni, M.; Comez, L.; Gallina, M.E.; Sassi, P.; Scarponi, F.; Fioretto, D.; Morresi, A. Light scattering spectra of water in trehalose aqueous solutions: Evidence for two different solvent relaxation processes. J. Phys. Chem. B 2009, 113, 7874–7878. [Google Scholar] [CrossRef]
- Lerbret, A.; Affouard, F.; Hedoux, A.; Krenzlin, S.; Siepmann, J.; Bellissent-Funel, M.-C.; Descamps, M. How strongly does trehalose interact with lysozyme in the solid state? Insights from molecular dynamics simulation and inelastic neutron scattering. J. Phys. Chem. B 2012, 116, 11103–11116. [Google Scholar] [CrossRef]
- Magazu, S.; Migliardo, F.; Gonzalez, M.A.; Mondelli, C. Inelastic neutron scattering study of dynamical properties of bioprotectant solutions against temperature. J. Non-Cryst. Solids 2012, 358, 2635–2640. [Google Scholar] [CrossRef]
- Faraone, A.; Magaza, S.; Lechner, R.E.; Longeville, S.; Maisano, G.; Majolino, D.; Migliardo, P.; Wanderlingh, U. Quasielastic neutron scattering from trehalose aqueous solutions. J. Chem. Phys. 2001, 115, 3281–3286. [Google Scholar] [CrossRef]
- Koper, I.; Bellissent-Funel, M.C.; Petry, W. Dynamics from picoseconds to nanoseconds of trehalose in aqueous solutions as seen by quasielastic neutron scattering. J. Chem. Phys. 2005, 122, 014514:1–014514:6. [Google Scholar]
- Magazu, S.; Villari, V.; Migliardo, P.; Maisano, G.; Telling, M.T.F. Diffusive dynamics of water in the presence of homologous disaccharides: A comparative study by quasi elastic neutron scattering. IV. J. Phys. Chem. B 2001, 105, 1851–1855. [Google Scholar] [CrossRef]
- Pagnotta, S.E.; McLain, S.E.; Soper, A.K.; Bruni, F.; Ricci, M.A. Water and trehalose: Flow much do they interact with each other? J. Phys. Chem. B 2010, 114, 4904–4908. [Google Scholar] [CrossRef]
- Winther, L.R.; Qvist, J.; Halle, B. Hydration and mobility of trehalose in aqueous solution. J. Phys. Chem. B 2012, 116, 9196–9207. [Google Scholar] [CrossRef]
- Ekdawi-Sever, N.; de Pablo, J.J.; Feick, E.; von Meerwall, E. Diffusion of sucrose and α,α-trehalose in aqueous solutions. J. Phys. Chem. A 2003, 107, 936–943. [Google Scholar] [CrossRef]
- Karger, N.; Ludemann, H.D. Temperature-dependence of the rotational mobility of the sugar and water-molecules in concentrated aqueous trehalose and sucrose solutions. Z. Naturforsch., C, J. Biosci. 1991, 46, 313–317. [Google Scholar]
- Rampp, M.; Buttersack, C.; Ludemann, H.D. c,T-dependence of the viscosity and the self-diffusion coefficients in some aqueous carbohydrate solutions. Carbohyd. Res. 2000, 328, 561–572. [Google Scholar] [CrossRef]
- Uedaira, H.; Ishimura, M.; Tsuda, S. Hydration of oligosaccharides. Bull. Chem. Soc. Jpn. 1990, 63, 3376–3379. [Google Scholar] [CrossRef]
- Poppe, L.; Vanhalbeek, H. NMR-spectroscopy of hydroxyl protons in supercooled carbohydrates. Nature Struct. Biology. 1994, 1, 215–216. [Google Scholar] [CrossRef]
- Magazu, S.; Maisano, G.; Migliardo, F.; Malomuzh, N.P.; Orlov, E.V. Transport and diffusion processes in trehalose-water solutions: Theory and experiments. Chem. Phys. 2006, 330, 90–100. [Google Scholar] [CrossRef]
- Magazu, S.; Maisano, G.; Migliardo, P.; Tettamanti, E.; Villari, V. Transport phenomena and anomalous glass-forming behaviour in α,α-trehalose aqueous solutions. Mol. Phys. 1999, 96, 381–387. [Google Scholar]
- Ribeiro, A.C.F.; Ortona, O.; Simoes, S.M.N.; Santos, C.I.A.V.; Prazeres, P.M.R.A.; Valente, A.J.M.; Lobo, V.M.M.; Burrows, H.D. Binary mutual diffusion coefficients of aqueous solutions of sucrose, lactose, glucose, and fructose in the temperature range from (298.15 to 328.15) K. J. Chem. Eng. Data 2006, 51, 1836–1840. [Google Scholar] [CrossRef]
- Ekdawi-Sever, N.C.; Conrad, P.B.; de Pablo, J.J. Molecular simulation of sucrose solutions near the glass transition temperature. J. Phys. Chem. A 2001, 105, 734–742. [Google Scholar] [CrossRef]
- Batta, G.; Kover, K.E. Heteronuclear coupling constants of hydroxyl protons in a water solution of oligosaccharides: Trehalose and sucrose. Carbohyd. Res. 1999, 320, 267–272. [Google Scholar] [CrossRef]
- Poppe, L.; Vanhalbeek, H. The rigidity of sucrose: Just an illusion. J. Am. Chem. Soc. 1992, 114, 1092–1094. [Google Scholar] [CrossRef]
- Sheng, S.Q.; Vanhalbeek, H. Evidence for a transient interresidue hydrogen bond in sucrose in aqueous solution obtained by rotating-frame exchange NMR spectroscopy under supercooled conditions. Biochem. Biophys. Res. Commun. 1995, 215, 504–510. [Google Scholar] [CrossRef]
- Fraser, R.R.; Kaufman, M.; Morand, P.; Govil, G. Stereochemical dependence of vicinal H-C-O-H coupling constants. Can. J. Chem. 1969, 47, 403–409. [Google Scholar]
- Brown, G.M.; Rohrer, D.C.; Berking, B.; Beevers, C.A.; Gould, R.O.; Simpson, R. The crystal structure of α,α-trehalose dihydrate from three independent X-ray determinations. Acta Crystallogr. B 1972, 28, 3145–3158. [Google Scholar] [CrossRef]
- Nagase, H.; Ogawa, N.; Endo, T.; Shiro, M.; Ueda, H.; Sakurai, M. Crystal structure of an anhydrous form of trehalose: Structure of water channels of trehalose polymorphism. J. Phys. Chem. B 2008, 112, 9105–9111. [Google Scholar] [CrossRef]
- Bekiroglu, S.; Sandstrom, A.; Kenne, L.; Sandstrom, C. Ab initio and NMR studies on the effect of hydration on the chemical shift of hydroxy protons in carbohydrates using disaccharides and water/methanol/ethers as model systems. Org. Biomol. Chem. 2004, 2, 200–205. [Google Scholar] [CrossRef]
- Ivarsson, I.; Sandstrom, C.; Sandstrom, A.; Kenne, L. 1H NMR chemical shifts of hydroxy protons in conformational analysis of disaccharides in aqueous solution. J. Chem. Soc. Perkin. Trans. 2 2000, 2147–2152. [Google Scholar]
- Sandstrom, C.; Baumann, H.; Kenne, L. The use of chemical shifts of hydroxy protons of oligosaccharides as conformational probes for NMR studies in aqueous solution. Evidence for persistent hydrogen bond interaction in branched trisaccharides. J. Chem. Soc. Perkin. Trans. 2 1998, 2385–2393. [Google Scholar] [CrossRef]
- Davies, D.B.; Christofides, J.C. Comparison of intramolecular hydrogen-bonding conformations of sucrose-containing oligosaccharides in solution and the solid state. Carbohyd. Res. 1987, 163, 269–274. [Google Scholar] [CrossRef]
- Brown, G.M.; Levy, H.A. Further refinement of structure of sucrose based on neutron-diffraction data. Acta Crystallogr. B-Struct. Sci. 1973, 29, 790–797. [Google Scholar] [CrossRef]
- Adams, B.; Lerner, L. Observation of hydroxyl protons of sucrose in aqueous-solution: No evidence for persistent intramolecular hydrogen-bonds. J. Am. Chem. Soc. 1992, 114, 4827–4829. [Google Scholar] [CrossRef]
- Wu, D.H.; Chen, A.D.; Johnson, C.S. An improved diffusion-ordered spectroscopy experiment incorporating bipolar-gradient pulses. J. Magn. Resonance. Ser. A 1995, 115, 260–264. [Google Scholar] [CrossRef]
- Liu, M.L.; Mao, X.A.; Ye, C.H.; Huang, H.; Nicholson, J.K.; Lindon, J.C. Improved watergate pulse sequences for solvent suppression in NMR spectroscopy. J. Magn. Reson. 1998, 132, 125–129. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Resonance Series A 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Sample Availability: Not available from authors, all compounds are commercially available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vilén, E.M.; Sandström, C. NMR Study on the Interaction of Trehalose with Lactose and Its Effect on the Hydrogen Bond Interaction in Lactose. Molecules 2013, 18, 9735-9754. https://doi.org/10.3390/molecules18089735
Vilén EM, Sandström C. NMR Study on the Interaction of Trehalose with Lactose and Its Effect on the Hydrogen Bond Interaction in Lactose. Molecules. 2013; 18(8):9735-9754. https://doi.org/10.3390/molecules18089735
Chicago/Turabian StyleVilén, Eric Morssing, and Corine Sandström. 2013. "NMR Study on the Interaction of Trehalose with Lactose and Its Effect on the Hydrogen Bond Interaction in Lactose" Molecules 18, no. 8: 9735-9754. https://doi.org/10.3390/molecules18089735




