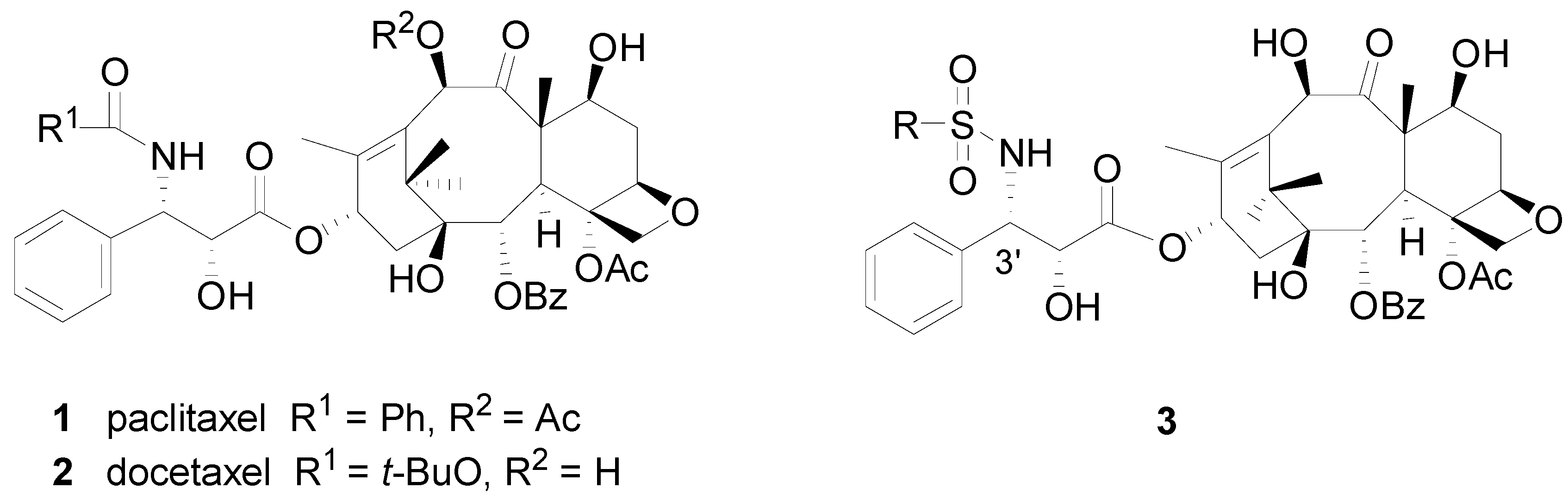
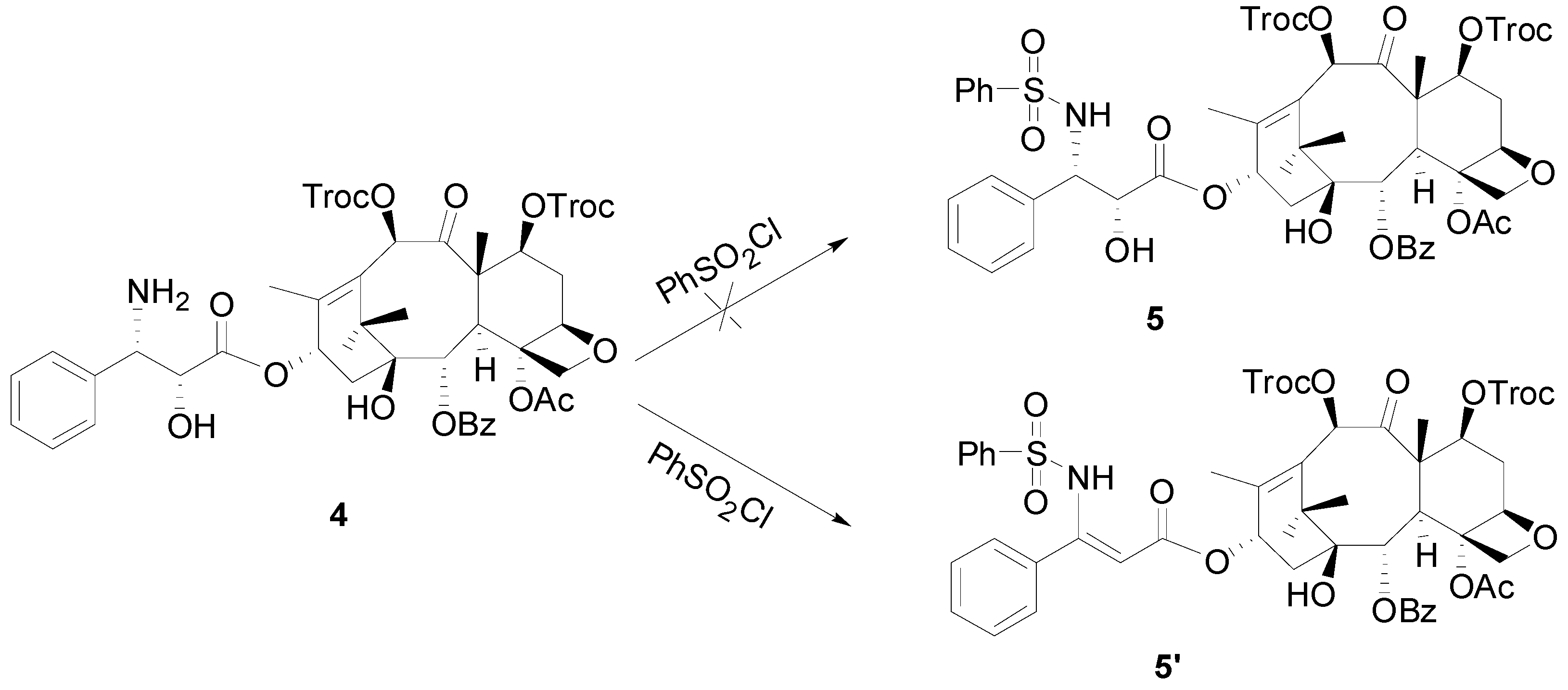
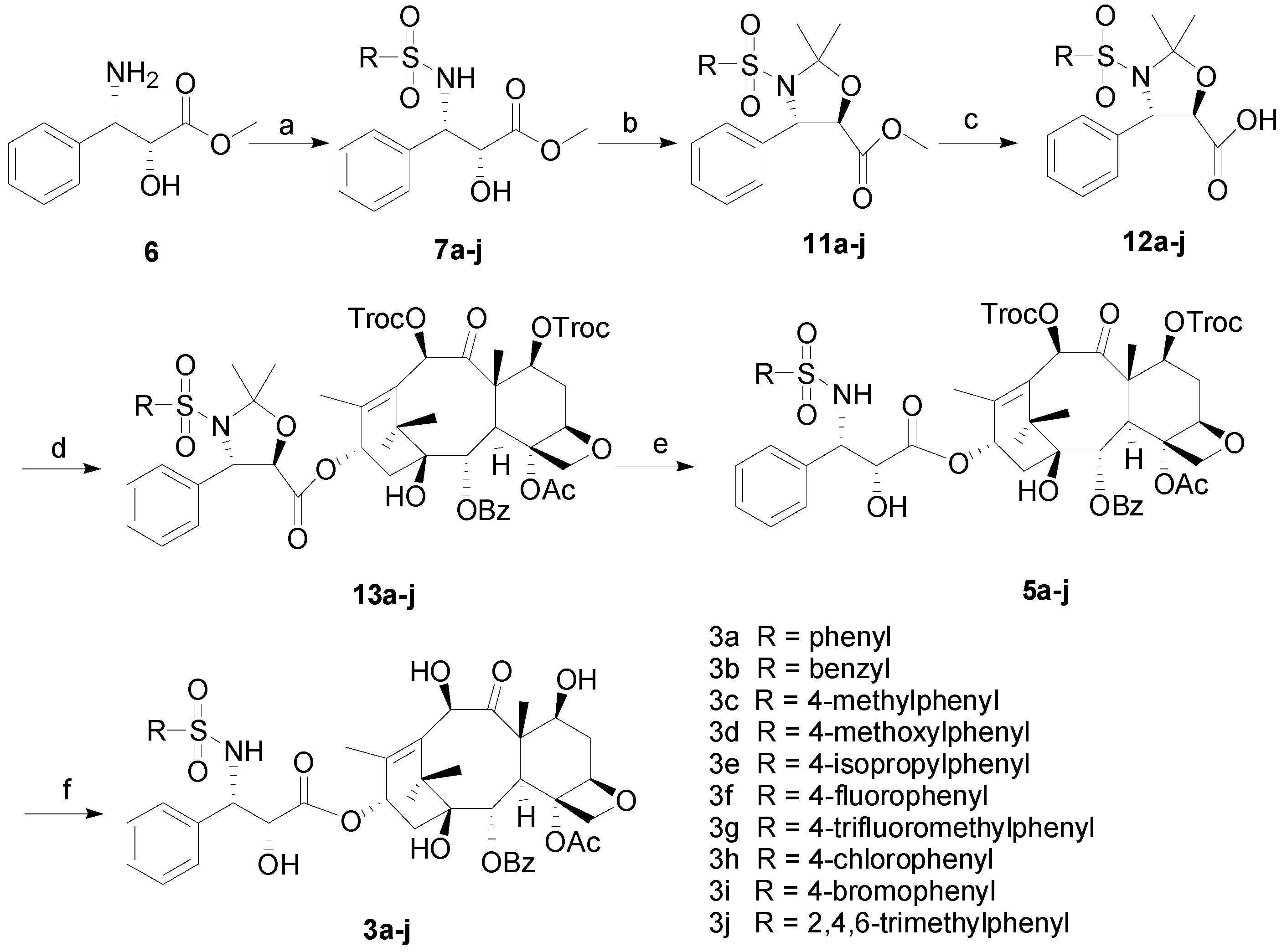
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of 7a–j
To a round-bottomed flask (2R,3S)-3-amino-2-hydroxy-3-phenyl-propionic acid methyl ester (6, 0.39 g, 2 mmol) was added into THF (15 mL), which was cooled to 0 oC. To this suspension was added Et3N (1.11 mL, 8 mmol), followed by the dropwise addition of phenylsulfonyl chloride (2.2 mmol). After further stirred at this temperature for 1 h, it was diluted with DCM (30 mL). The organic layer was washed with brine, dried over anhydrous Na2SO4 and filtered. Then the filtrate was evaporated and the residue was purified by column chromatography using petroleum ether/EtOAc (2/1) to afford the products 7a–j as white solids.
2-Hydroxy-3-benzenesulfonylamino-3-phenyl-propionic acid methyl ester (7a). Yield 59% (395 mg); 1H-NMR (CD3COCD3): δ 3.60 (s, 3H, OCH3), 4.35 (d, 1H, J = 3.2 Hz, 2-CH), 4.88 (d, 1H, J = 3.2 Hz, 3-CH), 7.14 (m, 3H, 3-Ph), 7.27 (m, 2H, 3-Ph), 7.35 (t, 2H, J = 7.6 Hz, m-PhSO2), 7.46 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.66 (t, 2H, J = 7.6 Hz, PhSO2); 13C-NMR (CD3COCD3): δ 172.72, 142.67, 139.31, 132.76, 129.44, 128.72, 128.26, 128.07, 127.63, 75.45, 60.92, 52.52; ESI-MS (m/z): 336 [M + H]+. HRMS (ESI) m/z calcd. for C16H17NO5SNa [M + Na]+: 358.0725, found 358.0704.
2-Hydroxy-3-phenylmethane sulfonylamino-3-phenyl-propionic acid methyl ester (7b). Yield 59% (412 mg); 1H-NMR (CD3COCD3): δ 3.69 (s, 3H, OCH3), 4.01 (d, 1H, J = 13.2 Hz, CH2 in Bn), 4.13 (d, 1H, J = 14.0 Hz, CH2 in Bn), 4.44 (d, 1H, J = 4.0 Hz, 2-CH), 4.93 (d, 1H, J = 3.6 Hz, 3-CH), 7.19 (m, 2H, 3-Ph), 7.27 (m, 3H, 3-Ph), 7.39 (m, 3H, Ph in Bn), 7.52 (m, 2H, Ph in Bn); 13C-NMR (CD3COCD3): 172.92, 140.33, 131.73, 130.88, 129.21, 129.03, 128.85, 128.66, 128.61, 75.63, 61.10, 60.41, 52.56; ESI-MS (m/z): 350 [M + H]+. HRMS (ESI) m/z calcd. for C17H19NO5SNa [M + Na]+: 372.0882, found 372.0859.
2-Hydroxy-3-(4-methyl benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7c). Yield 64% (448 mg); 1H-NMR (CD3COCD3): δ 2.31 (s, 3H, CH3 in Tosyl), 3.60 (s, 3H, OCH3), 4.34 (d, 1H, J = 2.8 Hz, 2-CH), 4.84 (d, 1H, J = 3.6 Hz, 3-CH), 7.27 (m, 5H, 3′-Ph), 7.26 (m, 2H, Ph in Tosyl), 7.53 (d, 2H, J = 8.0 Hz, Ph in Tosyl); 13C-NMR (CD3COCD3): 172.73, 143.39, 139.45, 129.92, 128.70, 128.29, 127.96, 127.73, 75.45, 60.87, 52.50, 21.29; ESI-MS (m/z): 350 [M + H]+. HRMS (ESI) m/z calcd. for C17H19NO5SNa [M + Na]+: 372.0882, found 372.0869.
2-Hydroxy-3-(4-methoxy benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7d). Yield 59% (432 mg); 1H-NMR (CD3COCD3): δ 3.63 (s, 3H, 3′-CH3), 3.81 (s, 3H, OCH3), 4.33 (d, 1H, J = 4.0 Hz, 2-CH), 4.83 (d, 1H, J = 3.2 Hz, 3-CH), 6.85 (m, 2H, PhSO2), 7.15 (m, 3H, 3-Ph), 7.26 (m, 2H, 3-Ph), 7.57 (m, 2H, PhSO2); 13C-NMR (CD3COCD3): 172.74, 163.31, 139.38, 134.30, 129.78, 128.94, 128.70, 128.29, 114.52, 75.50, 60.86, 55.98, 52.53; ESI-MS (m/z): 366 [M + H]+. HRMS (ESI) m/z calcd. for C17H19NO6SNa [M + Na]+: 388.0831, found 388.0837.
2-Hydroxy-3-(4-isopropyl benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7e). Yield 59% (445 mg); 1H-NMR (CD3COCD3): δ 1.19 (d, 6H, J = 3.2 Hz, 2CH3 in i-PrPh), 2.89 (m, 1H, CH in i-PrPh), 3.61 (s, 3H, OCH3), 4.34 (d, 1H, J = 3.2 Hz, 2-CH), 4.85 (d, 1H, J = 2.8 Hz, 3-CH), 7.12 (m, 3H, 3-Ph), 7.19 (d, 2H, J = 8.4 Hz, PhSO2), 7.23 (m, 2H, 3-Ph), 7.40 (m, 2H, PhSO2); 13C-NMR (CD3COCD3): 172.74, 154.05, 139.99, 139.20, 128.66, 128.31, 127.97, 127.87, 127.38, 75.44, 60.91, 52.51, 34.74, 23.93; ESI-MS (m/z): 378 [M + H]+. HRMS (ESI) m/z calcd. for C19H23NO5SNa [M + Na]+: 400.1195, found 400.1180.
2-Hydroxy-3-(4-fluoro benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7f). Yield 49% (346 mg); 1H-NMR (CD3COCD3): δ 3.66 (s, 3H, OCH3), 4.35 (d, 1H, J = 2.8 Hz, 2-CH), 4.88 (d, 1H, J = 3.2 Hz, 3-CH), 7.08 (t, 2H, J = 8.8 Hz, PhSO2), 7.15 (m, 3H, 3-Ph), 7.26 (m, 2H, 3-Ph), 7.68 (m, 2H, PhSO2); 13C-NMR (CD3COCD3): 172.68, 166.54, 164.06, 138.94, 130.66, 130.57, 128.73, 128.37, 128.10, 116.41, 116.18, 75.41, 61.01, 52.53; ESI-MS (m/z): 354 [M + H]+. HRMS (ESI) m/z calcd. for C16H16FNO5SNa [M + Na]+: 376.0631, found 376.0618.
2-Hydroxy-3-(4-trifluoromethyl benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7g). Yield 19% (153 mg); 1H-NMR (CD3COCD3): δ 3.65 (s, 3H, OCH3), 4.37 (d, 1H, J = 3.2 Hz, 2-CH), 4.91 (d, 1H, J = 3.2 Hz, 3-CH), 7.11 (m, 3H, 3-Ph), 7.23 (m, 2H, 3-Ph), 7.66 (d, 2H, J = 8.4 Hz, PhSO2), 7.82 (d, 2H, J = 8.0 Hz, PhSO2); 13C-NMR (CD3COCD3): 172.62, 146.30, 138.65, 133.69, 133.37, 128.73, 128.54, 128.43, 128.16, 126.52, 126.48, 126.44, 125.90, 123.20, 75.33, 61.19, 52.51; ESI-MS (m/z): 404 [M + H]+. HRMS (ESI) m/z calcd. for C17H16F3NO5SNa [M + Na]+: 426.0599, found 426.0606.
2-Hydroxy-3-(4-chloro benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7h). Yield 32% (236 mg); 1H-NMR (CD3COCD3): δ 3.65 (s, 3H, OCH3), 4.36 (d, 1H, J = 3.2 Hz, 2-CH), 4.88 (d, 1H, J = 3.2 Hz, 3-CH), 7.15 (m, 3H, 3-Ph), 7.26 (m, 2H, 3-Ph), 7.35 (d, 2H, J = 8.8 Hz, PhSO2), 7.62 (d, 2H, J = 8.8 Hz, PhSO2); 13C-NMR (CD3COCD3): 172.65, 141.42, 138.98, 138.36, 129.49, 128.74, 128.39, 128.10, 75.38, 61.05, 52.51; ESI-MS (m/z): 370 [M + H]+. HRMS (ESI) m/z calcd. for C16H16ClNO5SNa [M + Na]+: 392.0335, found 392.0342.
2-Hydroxy-3-(4-bromo benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7i). Yield 35% (290 mg); 1H-NMR (CD3COCD3): δ 3.64 (s, 3H, OCH3), 4.36 (d, 1H, J = 3.2 Hz, 2-CH), 4.88 (d, 1H, J = 3.2 Hz, 3-CH), 7.16 (m, 3H, 3-Ph), 7.26 (m, 2H, 3-Ph), 7.51 (m, 4H, PhSO2); 13C-NMR (CD3COCD3): 172.65, 141.88, 138.98, 132.51, 129.60, 128.75, 128.40, 128.09, 126.87, 75.37, 61.06, 52.51; ESI-MS (m/z): 413 [M + H]+. HRMS (ESI) m/z calcd. For C16H16BrNO5SNa [M + Na]+: 435.9830, found 435.9807.
2-Hydroxy-3-(2,4,6-trimethyl benzenesulfonylamino)-3-phenyl-propionic acid methyl ester (7j). Yield 50% (377 mg); 1H-NMR (CD3COCD3): δ 2.23 (s, 3H, CH3 in PhSO2), 2.53 (s, 6H, CH3 in PhSO2), 3.45 (s, 3H, OCH3), 4.32 (d, 1H, J = 3.2 Hz, 2-CH), 4.72 (d, 1H, J = 3.2 Hz, 3-CH), 6.90 (s, 2H, PhSO2), 7.20 (m, 3H, 3-Ph), 7.31 (m, 2H, 3-Ph); 13C-NMR (CD3COCD3): 172.67, 142.56, 139.92, 139.49, 136.23, 132.43, 128.70, 128.11, 128.05, 75.18, 60.45, 52.37, 23.09, 20.74; ESI-MS (m/z): 378 [M + H]+. HRMS (ESI) m/z calcd. for C19H23NO5SNa [M + Na]+: 400.1195, found 400.1180.
3.2.2. General Procedure for the Synthesis of 11a–j
To a stirred solution of 7a–j (0.45 mmol) and PPTs (113 mg, 0.045 mmol) in anhydrous toluene (8 mL) was added 2-methoxypropene (0.129 mL, 1.35 mmol). The reaction mixture was warmed to 85 °C , and further stirred for 2 h at this temperature. After cooled down to room temperature, the mixture was diluted with EtOAc (50 mL). The organic layer was washed with brine, dried over anhydrous Na2SO4 and filtered. The filtrate was evaporated and the residue was purified by silica gel column chromatography using petroleum ether/EtOAc (2/1) to obtain products 11a–j as colorless liquids.
2,2-Dimethyl-3-benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11a). Yield 97% (164 mg); 1H-NMR (CD3COCD3): δ 1.69 (s, 3H, i-Pr), 1.81 (s, 3H, i-Pr), 3.66 (s, 3H, OCH3), 4.62 (d, 1H, J = 4.8 Hz, 2-CH), 5.27 (d, 1H, J = 4.8 Hz, 3-CH), 7.22 (m, 3H, 3′-Ph), 7.32 (m, 2H, 3′-Ph), 7.42 (t, 2H, J = 7.6 Hz, m-PhSO2), 7.55 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.62 (t, 2H, J = 7.6 Hz, o-PhSO2); 13C-NMR (CD3COCD3): 171.03, 142.02, 139.51, 133.41, 129.60, 129.14, 128.76, 128.46, 128.32, 100.78, 82.50, 66.19, 52.76, 27.31; ESI-MS (m/z): 376 [M + H]+. HRMS (ESI) m/z calcd. for C19H21NO5SNa [M + Na]+: 398.1038, found 398.1024.
2,2-Dimethyl-3-phenylmethane sulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11b). Yield 92% (161 mg); 1H-NMR (CD3COCD3): δ 1.60 (s, 3H, i-Pr), 1.73 (s, 3H, i-Pr), 3.79 (s, 3H, OCH3), 3.79 (d, 1H, J = 14.0 Hz, CH2 in Bn), 4.10 (d, 1H, J = 13.6 Hz, CH2 in Bn), 4.79 (d, 1H, J = 4.0 Hz, 2-CH), 5.30 (d, 1H, J = 4.4 Hz, 3-CH), 7.25 (m, 2H, 3-Ph), 7.33 (m, 3H, 3-Ph), 7.40 (m, 1H, Ph in Bn), 7.48 (m, 2H, Ph in Bn), 7.58 (m, 2H, Ph in Bn); 13C-NMR (CD3COCD3): 171.57, 140.54, 131.98, 130.12, 129.58, 129.30, 129.14, 128.97, 100.28, 82.18, 65.65, 61.03, 52.88, 28.95, 27.74; ESI-MS (m/z): 390 [M + H]+; Anal. calcd. for C20H23NO5S: C, 61.68; H, 5.95; N, 3.60. Found: C, 61.73; H, 6.05; N, 3.52.
2,2-Dimethyl-3-(4-methyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11c). Yield 80% (140 mg); 1H-NMR (CD3COCD3): δ 1.68 (s, 3H, i-Pr), 1.79 (s, 3H, i-Pr), 2.36 (s, 3H, CH3 in tosyl), 3.66 (s, 3H, OCH3), 4.60 (d, 1H, J = 4.8 Hz, 2-CH), 5.24 (d, 1H, J = 4.8 Hz, 3-CH), 7.23 (m, 5H, 3′-Ph), 7.32 (m, 2H, Ph in tosyl), 7.50 (d, 2H, J = 8.4 Hz, Ph in tosyl); 13C-NMR (CD3COCD3): 171.04, 144.23, 139.71, 139.12, 130.07, 129.10, 128.62, 128.43, 128.42, 100.68, 82.48, 66.20, 52.75, 27.22, 21.37; ESI-MS (m/z): 390 [M + H]+. HRMS (ESI) m/z calcd. for C20H23NO5SNa [M + Na]+: 412.1195, found 412.1196.
2,2-Dimethyl-3-(4-methoxy)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11d). Yield 99% (180 mg); 1H-NMR (CD3COCD3): δ 1.69 (s, 3H, i-Pr), 1.80 (s, 3H, i-Pr), 3.67 (s, 3H, 3-CH3), 3.85 (s, 3H, OCH3), 4.58 (d, 1H, J = 4.8 Hz, 2-CH), 5.21 (d, 1H, J = 4.8 Hz, 3-CH), 6.89 (m, 2H, PhSO2), 7.22 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.53 (m, 2H, PhSO2); 13C-NMR (CD3COCD3): 171.06, 163.73, 139.67, 133.52, 130.57, 129.08, 128.63, 128.38, 114.63, 100.65, 82.51, 66.12, 60.57, 56.09, 52.73, 27.15; ESI-MS (m/z): 406 [M + H]+; Anal. calcd. for C20H23NO6S: C, 59.24; H, 5.72; N, 3.45. Found: C, 59.21; H, 5.70; N, 3.49.
2,2-Dimethyl-3-(4-isopropyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11e). Yield 97% (182 mg); 1H-NMR (CD3COCD3): δ 1.22 (d, 6H, J = 7.4 Hz, 2CH3 in i-PrPh), 1.71 (s, 3H, i-Pr), 1.83 (s, 3H, i-Pr), 2.94 (m, 1H, CH in i-PrPh), 3.66 (s, 3H, OCH3), 4.59 (d, 1H, J = 4.8 Hz, 2-CH), 5.21 (d, 1H, J = 5.2 Hz, 3-CH), 7.19 (m, 3H, 3-Ph), 7.24 (d, 2H, J = 8.8 Hz, PhSO2), 7.26 (m, 2H, 3-Ph), 7.49 (d, 2H, J = 8.8 Hz, PhSO2); 13C-NMR (CD3COCD3): 170.14, 153.83, 138.44, 138.35, 128.18, 127.91, 127.68, 127.57, 126.60, 99.98, 81.68, 78.33, 65.24, 51.82, 33.87, 26.42, 23.03, 22.98; ESI-MS (m/z): 418 [M + H]+. HRMS (ESI) m/z calcd. for C22H27NO5SNa [M + Na]+: 440.1508, found 440.1501.
2,2-Dimethyl-3-(4-fluoro)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11f). Yield 81% (143 mg); 1H-NMR (CD3COCD3): δ 1.71 (s, 3H, i-Pr), 1.83 (s, 3H, i-Pr), 3.69 (s, 3H, OCH3), 4.62 (d, 1H, J = 4.8 Hz, 2-CH), 5.23 (d, 1H, J = 4.8 Hz, 3-CH), 7.14 (t, 2H, J = 8.8 Hz, PhSO2), 7.22 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.63 (m, 2H, PhSO2); 13C-NMR (CD3COCD3): 171.02, 166.85, 164.35, 139.09, 138.26, 131.43, 131.33, 129.17, 128.82, 128.60, 116.63, 116.40, 100.97, 82.52, 66.07, 52.77, 27.38; ESI-MS (m/z): 394 [M + H]+. HRMS (ESI) m/z calcd. for C19H20FNO5SNa [M + Na]+: 416.0944, found 416.0936.
2,2-Dimethyl-3-(4-trifluoromethyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11g). Yield 99% (197 mg); 1H-NMR (CD3COCD3): δ 1.74 (s, 3H, i-Pr), 1.87 (s, 3H, i-Pr), 3.69 (s, 3H, OCH3), 4.65 (d, 1H, J = 5.2 Hz, 2-CH), 5.26 (d, 1H, J = 4.8 Hz, 3-CH), 7.19 (m, 3H, 3-Ph), 7.26 (m, 2H, 3-Ph), 7.70 (d, 2H, J = 8.4 Hz, PhSO2), 7.76 (d, 2H, J = 8.0 Hz, PhSO2); 13C-NMR (CD3COCD3): 170.90, 145.58, 138.51, 134.14, 129.21, 129.17, 128.95, 128.78, 126.71, 126.68, 126.64, 126.60, 101.24, 82.49, 66.11, 52.79, 27.67; ESI-MS (m/z): 444 [M + H]+; Anal. calcd. for C20H20F3NO5S: C, 54.17; H, 4.55; N, 3.16. Found: C, 54.31; H, 4.51; N, 3.22.
2,2-Dimethyl-3-(4-chloro)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11h). Yield 93% (171 mg); 1H-NMR (CD3COCD3): δ 1.71 (s, 3H, i-Pr), 1.83 (s, 3H, i-Pr), 3.69 (s, 3H, OCH3), 4.63 (d, 1H, J = 5.2 Hz, 2-CH), 5.24 (d, 1H, J = 5.2 Hz, 3-CH), 7.24 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.41 (d, 2H, J = 8.8 Hz, PhSO2), 7.56 (d, 2H, J = 8.0 Hz, PhSO2); 13C-NMR (CD3COCD3): 170.99, 140.73, 139.10, 139.02, 130.15, 129.69, 129.21, 128.83, 128.66, 101.01, 82.50, 66.11, 52.79, 27.46; ESI-MS (m/z): 410 [M + H]+. HRMS (ESI) m/z calcd for C19H20ClNO5SNa [M + Na]+: 432.0648, found 432.0653.
2,2-Dimethyl-3-(4-bromo)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11i). Yield 99% (202 mg); 1H-NMR (CD3COCD3): δ 1.71 (s, 3H, i-Pr), 1.83 (s, 3H, i-Pr), 3.69 (s, 3H, OCH3), 4.63 (d, 1H, J = 5.2 Hz, 2-CH), 5.23 (d, 1H, J = 4.8 Hz, 3-CH), 7.24 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.49 (d, 2H, J = 8.8 Hz, PhSO2), 7.57 (d, 2H, J = 8.8 Hz, PhSO2); 13C-NMR (CD3COCD3): 170.96, 141.17, 138.99, 132.70, 130.20, 129.21, 128.81, 128.65, 127.67, 101.00, 82.48, 66.10, 52.78, 27.46; ESI-MS (m/z): 455 [M + H]+. HRMS (ESI) m/z calcd. for C19H20BrNO5SNa [M + Na]+: 476.0143, found 476.0156.
2,2-Dimethyl-3-(2,4,6-trimethyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid methyl ester (11j). Yield 81% (152 mg); 1H-NMR (CD3COCD3): δ 1.82 (s, 3H, i-Pr), 1.92 (s, 3H, i-Pr), 2.10 (s, 3H, CH3 in PhSO2), 2.51 (s, 6H, CH3 in PhSO2), 3.74 (s, 3H, OCH3), 4.47 (d, 1H, J = 5.6 Hz, 2-CH), 4.99 (d, 1H, J = 6.0 Hz, 3-CH), 6.63 (s, 2H, PhSO2), 7.01 (m, 5H, 3-Ph); 13C-NMR (CD3COCD3): 171.11, 144.03, 140.69, 138.39, 134.00, 132.53, 128.67, 128.25, 127.46, 102.10, 83.12, 66.06, 52.72, 27.12, 23.21, 20.67; ESI-MS (m/z): 418 [M + H]+; Anal. calcd. for C22H27NO5S: C, 63.29; H, 6.52; N, 3.35. Found: C, 63.42; H, 6.48; N, 3.39.
3.2.3. General Procedure for the Synthesis of 12a–j
To a stirred solution of 11a–j (0.437 mmol) in a mixture of solvent (THF:H2O = 6 mL:2 mL) at 0 °C was added LiOH•H2O (37 mg, 0.87 mmol). The resulting mixture was warmed to room temperature and further stirred for 1 h. Then the pH of the mixture was adjusted to 2~3 by adding 1N HCl solution. After extracted with CH2Cl2 three times, the combined organic phase was dried over anhydrous Na2SO4 and evaporated to afford the colorless liquid products 12a–j.
2,2-Dimethyl-3-benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12a). Yield 97% (154 mg); 1H-NMR (CD3COCD3): δ 1.71 (s, 3H, i-Pr), 1.83 (s, 3H, i-Pr), 4.56 (d, 1H, J = 4.8 Hz, 2-CH), 5.25 (d, 1H, J = 4.4 Hz, 3-CH), 7.21 (m, 3H, 3′-Ph), 7.31 (m, 2H, 3′-Ph), 7.39 (t, 2H, J = 7.6 Hz, m-PhSO2), 7.53 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.60 (t, 2H, J = 7.6 Hz, o-PhSO2); 13C-NMR (CD3COCD3) δ 171.48, 142.04, 139.68, 133.34, 129.55, 129.12, 128.69, 128.50, 128.305, 100.72, 82.50, 66.24, 27.41; ESI-MS (m/z): 362 [M + H]+. HRMS (ESI) m/z calcd. for C18H19NO5SNa [M + Na]+: 384.0882, found 384.0888.
2,2-Dimethyl-3-phenylmethane sulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12b). Yield 97% (160 mg); 1H-NMR (CD3COCD3): δ 1.61 (s, 3H, i-Pr), 1.76 (s, 3H, i-Pr), 3.79 (d, 1H, J = 14.0 Hz, CH2 in Bn), 4.10 (d, 1H, J = 14.0 Hz, CH2 in Bn), 4.76 (d, 1H, J = 4.0 Hz, 2-CH), 5.30 (d, 1H, J = 4.0 Hz, 3-CH), 7.24 (m, 3H, 3-Ph), 7.33 (m, 2H, 3-Ph), 7.40 (m, 2H, Ph in Bn), 7.59 (m, 3H, Ph in Bn); 13C-NMR (CD3COCD3) δ 172.03, 140.75, 131.99, 131.74, 130.93, 130.15, 129.56, 129.24, 129.15, 129.04, 129.00, 128.83, 128.62, 128.55, 100.23, 82.12, 75.05, 65.72, 27.81; ESI-MS (m/z): 376 [M + H]+. HRMS (ESI) m/z calcd. for C19H21NO5SNa [M + Na]+: 398.1038, found 398.1031.
2,2-Dimethyl-3-(4-methyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12c). Yield 96% (158 mg); 1H-NMR (CD3COCD3): δ 1.70 (s, 3H, i-Pr), 1.82 (s, 3H, i-Pr), 2.35 (s, 3H, CH3 in Tosyl), 4.55 (d, 1H, J = 5.2 Hz, 2-CH), 5.22 (d, 1H, J = 5.2 Hz, 3-CH), 7.22 (m, 5H, Ph in Tosyl), 7.30 (m, 2H, Ph in Tosyl), 7.48 (d, 2H, Ph in Tosyl); 13C-NMR (CD3COCD3) δ 171.47, 144.15, 139.91, 139.12, 130.04, 129.09, 128.55, 128.45, 128.44, 100.63, 82.49, 66.23, 27.31, 21.37; ESI-MS (m/z): 376 [M + H]+. HRMS (ESI) m/z calcd. for C19H21NO5SNa [M + Na]+: 398.1038, found 398.1031.
2,2-Dimethyl-3-(4-methoxy)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12d). Yield 99% (170 mg); 1H-NMR (CD3COCD3): δ 1.74 (s, 3H, i-Pr), 1.88 (s, 3H, i-Pr), 3.70 (s, 3H, 3-CH3), 4.67 (d, 1H, J = 4.8 Hz, 2-CH), 5.29 (d, 1H, J = 5.2 Hz, 3-CH), 7.21 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.80 (d, 2H, J = 9.2 Hz, PhSO2), 8.19 (d, 2H, J = 8.8 Hz, PhSO2); 13C-NMR (CD3COCD3) δ 170.87, 150.65, 147.26, 138.47, 129.81, 129.30, 129.06, 128.9, 124.68, 101.32, 82.43, 66.12, 52.85, 27.78; ESI-MS (m/z): 392 [M + H]+. HRMS (ESI) m/z calcd. for C19H21NO6SNa [M + Na]+: 414.0987, found 414.0998.
2,2-Dimethyl-3-(4-isopropyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12e). Yield 95% (168 mg); 1H-NMR (CD3COCD3): δ 1.21 (d, 3H, J = 1.6 Hz, CH3 in i-PrPh), 1.22 (d, 3H, J = 1.2 Hz, CH3 in i-PrPh), 1.74 (s, 3H, CH3 in i-Pr), 1.85 (s, 3H, CH3 in i-Pr), 2.92 (m, 1H, CH in i-PrPh), 4.53 (d, 1H, J = 5.2 Hz, 2-CH), 5.19 (d, 1H, J = 5.2 Hz, 3-CH), 7.16 (m, 3H, 3-Ph), 7.20 (d, 2H, J = 8.4 Hz, PhSO2), 7.23 (m, 2H, 3-Ph), 7.46 (m, 2H, PhSO2); 13C-NMR (CD3COCD3) δ 171.48, 154.64, 139.36, 139.33, 129.06, 128.64, 128.55, 128.54, 127.45, 100.83, 82.59, 66.20, 34.76, 27.46, 23.95, 23.86; ESI-MS (m/z): 404 [M + H]+. HRMS (ESI) m/z calcd. for C21H25NO5SNa [M + Na]+: 426.1351, found 426.1339.
2,2-Dimethyl-3-(4-fluoro)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12f). Yield 81% (135 mg); 1H-NMR (CD3COCD3): δ 1.73 (s, 3H, i-Pr), 1.84 (s, 3H, i-Pr), 4.58 (d, 1H, J = 5.2 Hz, 2-CH), 5.23 (d, 1H, J = 5.2 Hz, 3-CH), 7.12 (t, 2H, J = 8.8 Hz, PhSO2), 7.22 (m, 3H, 3-Ph), 7.28 (m, 2H, 3-Ph), 7.63 (m, 2H, PhSO2); 13C-NMR (CD3COCD3) δ 171.45, 166.83, 164.32, 139.32, 138.29, 138.26, 131.43, 131.33, 129.16, 128.76, 128.62, 116.60, 116.38, 100.93, 82.50, 66.12, 27.47; ESI-MS (m/z): 380 [M + H]+. HRMS (ESI) m/z calcd. for C18H18FNO5SNa [M + Na]+: 402.0787, found 402.0782.
2,2-Dimethyl-3-(4-trifluoromethyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12g). Yield 78% (147 mg); 1H-NMR (CD3COCD3): δ 1.77 (s, 3H, i-Pr), 1.88 (s, 3H, i-Pr), 4.64 (d, 1H, J = 5.2 Hz, 2-CH), 5.29 (d, 1H, J = 4.8 Hz, 3-CH), 7.21 (m, 3H, 3-Ph), 7.30 (m, 2H, 3-Ph), 7.81 (d, 2H, J = 8.8 Hz, PhSO2), 8.18 (d, 2H, J = 9.2 Hz, PhSO2); 13C-NMR (CD3COCD3) δ 171.32, 150.62, 147.27, 138.73, 129.82, 129.28, 128.99, 128.89, 124.68, 101.26, 82.38, 66.18, 27.83; ESI-MS (m/z): 430 [M + H]+. HRMS (ESI) m/z calcd. for C19H18F3NO5SNa [M + Na]+: 452.0755, found 452.0737.
2,2-Dimethyl-3-(4-chloro)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12h). Yield 100% (173 mg); 1H-NMR (CD3COCD3): δ 1.73 (s, 3H, i-Pr), 1.84 (s, 3H, i-Pr), 4.59 (d, 1H, J = 5.2 Hz, 2-CH), 5.24 (d, 1H, J = 4.8 Hz, 3-CH), 7.23 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.39 (d, 2H, J = 8.8 Hz, PhSO2), 7.56 (d, 2H, J = 8.0 Hz, PhSO2); 13C-NMR (CD3COCD3) δ 171.40, 140.74, 139.26, 139.04, 130.14, 129.66, 129.19, 128.76, 128.67, 100.95, 82.46, 66.14, 27.54; ESI-MS (m/z): 396 [M + H]+. HRMS (ESI) m/z calcd. for C18H18ClNO5SNa [M + Na]+: 418.0492, found 418.0504.
2,2-Dimethyl-3-(4-bromo)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12i). Yield 99% (191 mg); 1H-NMR (CD3COCD3): δ 1.73 (s, 3H, i-Pr), 1.84 (s, 3H, i-Pr), 4.59 (d, 1H, J = 4.8 Hz, 2-CH), 5.23 (d, 1H, J = 5.2 Hz, 3-CH), 7.23 (m, 3H, 3-Ph), 7.29 (m, 2H, 3-Ph), 7.48 (d, 2H, J = 8.8 Hz, PhSO2), 7.55 (d, 2H, J = 8.8 Hz, PhSO2); 13C-NMR (CD3COCD3) δ 171.40, 141.20, 139.22, 132.67, 130.20, 129.20, 128.73, 128.70, 127.62, 100.93, 82.45, 66.14, 27.54; ESI-MS (m/z): 439 [M + H]+. HRMS (ESI) m/z calcd. for C18H18BrNO5SNa [M + Na]+: 461.9987, found 461.9997.
2,2-Dimethyl-3-(2,4,6-trimethyl)benzenesulfonyl-4-phenyl-oxazolidine-5-carboxylic acid (12j). Yield 99% (175 mg); 1H-NMR (CD3COCD3): δ 1.85 (s, 3H, i-Pr), 1.93 (s, 3H, i-Pr), 2.09 (s, 3H, CH3 in PhSO2), 2.52 (s, 6H, CH3 in PhSO2), 4.44 (d, 1H, J = 5.6 Hz, 2-CH), 5.02 (d, 1H, J = 5.6 Hz, 3-CH), 6.63 (s, 2H, PhSO2), 7.01 (m, 5H, 3-Ph); 13C-NMR (CD3COCD3) δ 171.58, 143.99, 140.68, 138.84, 134.01, 132.52, 128.65, 128.15, 127.39, 102.05, 83.10, 66.04, 27.20, 23.21, 20.68. ESI-MS (m/z): 404 [M + H]+. HRMS (ESI) m/z calcd for C21H25NO5SNa [M + Na]+: 426.1351, found 426.1345.
3.2.4. General Procedure for the Synthesis of 13a–j
To a solution of anhydrous toluene (60 mL) were added 12a–j (0.49 mmol), 7,10-ditroc-10-DAB (0.23 g, 0.24 mmol), DCC (0.15 g, 0.75 mmol) and DMAP (15 mg, 0.13 mmol). The resulting mixture was stirred at 85 °C for 3 h. After the completion, the reaction mixture was washed with brine (30 mL × 3), dried over Na2SO4, filtered and concentrated in vacuo. The obtained residue was purified by silica gel flash chromatography column (petroleum ether/ethyl acetate: 4/1) to afford 13a–j as white solids.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13a). Yield 96% (285 mg); mp 155–157 °C; 1H-NMR (CDCl3): δ 1.17 (s, 3H, 17-CH3), 1.24 (s, 3H, 16-CH3), 1.82 and 1.87 (2s, 6H, i-Pr), 1.95 (s, 3H, 19-CH3), 1.97 (s, 3H, 18-CH3), 1.99 (s, 3H, OAc), 2.13 (m, 2H, 14-CH2), 2.03 and 2.59 (2m, 2H, 6-CH2), 3.87 (d, 1H, J = 7.2 Hz, 3-CH), 4.11 and 4.27 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.50 (d, 1H, J = 6.0 Hz, 2′-CH), 4.60 and 4.91 (2d, 2H, J = 12.0 Hz, Troc), 4.78 (s, 2H, Troc), 4.90 (d, 1H, J = 7.6 Hz, 5-CH), 5.23 (d, 1H, J = 6.4 Hz, 3′-CH), 5.56 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.65 (d, 1H, J = 7.2 Hz, 2-CH), 6.21 (t, 1H, J = 8.4 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 7.15 (m, 2H, 3′-Ph), 7.21 (m, 3H, 3′-Ph), 7.26 (t, 2H, J = 7.6 Hz, m-PhSO2), 7.41 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.46 (d, 2H, J = 7.2 Hz, o-PhSO2), 7.47 (t, 2H, J = 7.6 Hz, m-OBz), 7.62 (t, 1H, J = 7.6 Hz, p-OBz), 8.01 (d, 2H, J = 7.2 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 170.14, 169.78, 166.84, 153.21, 142.48, 140.58, 136.95, 133.87, 132.41, 132.02, 130.05, 129.00, 128.67, 128.58, 128.46, 128.33, 128.00, 127.50, 100.95, 94.19, 83.68, 81.99, 80.43, 79.01, 78.88, 74.23, 71.49, 65.27, 58.46, 56.09, 49.21, 46.90, 43.07, 35.38, 33.91, 33.17, 28.73, 27.18, 26.23, 25.60, 24.92, 21.58, 21.03, 18.43, 14.81, 10.72. Anal. calcd. for C53H55Cl6NO18S: C, 51.39; H, 4.48; N, 1.13. Found: C, 51.52; H, 4.51; N, 1.15.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-phenyl methane sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13b). Yield 94% (282 mg); mp 154–156 °C; 1H-NMR (CDCl3): δ 1.20 (s, 3H, 17-CH3), 1.28 (s, 3H, 16-CH3), 1.61 (s, 3H, 19-CH3), 1.84 and 1.85 (2s, 6H, i-Pr), 2.08 (s, 3H, 18-CH3), 2.10 (s, 3H, OAc), 2.22 (m, 2H, 14-CH2), 2.04 and 2.62 (2m, 2H, 6-CH2), 3.73 (2d, 2H, J = 13.6 Hz, 3″-CH2), 3.93 (d, 1H, J = 7.2 Hz, 3-CH), 4.15 and 4.32 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.70 (d, 1H, J = 5.2 Hz, 2′-CH), 4.62 and 4.94 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (dd, 2H, J = 12.8, 12.0 Hz, Troc), 4.94 (d, 1H, J = 9.2 Hz, 5-CH), 5.29 (d, 1H, J = 5.2 Hz, 3′-CH), 5.61 (dd, 1H, J = 10.6, 7.0 Hz, 2-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 6.27 (t, 1H, J = 8.6 Hz, 13-CH), 6.27 (s, 1H, 10-CH), 7.21 (m, 2H, 3′-Ph), 7.35 (m, 3H, 3′-Ph), 751 (t, 2H, J = 8.0 Hz, m-OBz), 7.51 (m, 2H, 3″-Ph), 7.56 (m, 2H, 3″-Ph), 7.65 (t, 1H, J = 7.6 Hz, p-OBz), 8.05 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.67, 171.18, 170.18, 170.04, 166.86, 153.22, 153.19, 142.43, 138.48, 133.91, 132.07, 130.99, 130.07, 129.21, 129.14, 128.99, 128.80, 128.71, 128.59, 128.50, 128.17, 100.50, 94.19, 83.70, 81.67, 80.48, 79.02, 78.91, 74.24, 71.66, 64.89, 61.36, 60.41, 56.10, 49.31, 46.94, 43.10, 35.45, 33.83, 33.19, 29.70, 27.98, 27.77, 26.23, 25.57, 24.89, 2, 21.65, 21.06, 21.03, 14.96, 14.21, 10.74. Anal. calcd. for C54H57Cl6NO18S: C, 51.77; H, 4.59; N, 1.12. Found: C, 51.88; H, 4.65; N, 1.18.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-methyl) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13c). Yield 85% (255 mg); mp 154–156 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.84 and 1,87 (2s, 6H, i-Pr), 1.94 (s, 3H, 19-CH3), 1.99 (s, 3H, 18-CH3), 2.00 (s, 3H, OAc), 2.16 (m, 2H, 14-CH2), 2.36 (s, 3H, 4″-CH3), 2.05 and 2.61 (2m, 2H, 6-CH2), 3.89 (d, 1H, J = 6.8 Hz, 3-CH), 4.13 and 4.29 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.52 (d, 1H, J = 6.8 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (dd, 2H, J = 14.4, 12.0 Hz, Troc), 4.92 (d, 1H, J = 7.6 Hz, 5-CH), 5.21 (d, 1H, J = 6.8 Hz, 3′-CH), 5.58 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.67 (d, 1H, J = 7.2 Hz, 2-CH), 6.22 (t, 1H, J = 8.8 Hz, 13-CH), 6.25 (s, 1H, 10-CH), 7.07 (d, 2H, J = 8.4 Hz, m-PhSO2), 7.20 (m, 2H, 3′-Ph), 7.26 (m, 3H, 3′-Ph), 7.39 (d, 2H, J = 8.4 Hz, o-PhSO2), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.65 (t, 1H, J = 7.4 Hz, p-OBz), 8.04 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 171.15, 169.81, 166.85, 153.21, 153.20, 143.32, 142.51, 137.57, 133.88, 131.99, 130.06, 129.10, 128.99, 130.06, 129.10, 128.99, 128.68, 128.53, 128.16, 127.92, 100.81, 94.21, 94.19, 83.67, 81.94, 80.42, 79.02, 78.90, 74.23, 71.45, 65.33, 60.39, 56.08, 46.90, 43.07, 35.37, 33.10, 33.17, 29.70, 28.90, 26.99, 26.23, 25.51, 24.82, 21.57, 21.44, 21.03, 14.78, 14.20, 10.72. Anal. calcd. for C54H57Cl6NO18S: C, 51.77; H, 4.59; N, 1.12. Found: C, 51.93; H, 4.63; N, 1.16.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-methoxy) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13d). Yield 95% (288 mg); mp 159–161 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.26 (s, 3H, 16-CH3), 1.83 and 1.87 (2s, 6H, i-Pr), 1.94 (s, 3H, 19-CH3), 1.99 (s, 3H, 18-CH3), 2.01 (s, 3H, OAc), 2.15 (m, 2H, 14-CH2), 2.05 and 2.61 (2m, 2H, 6-CH2), 3.82 (s, 3H, OCH3), 3.89 (d, 1H, J = 6.8 Hz, 3-CH), 4.13 and 4.28 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.51 (d, 1H, J = 6.8 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (dd, 2H, J = 14.0, 12.0 Hz, Troc), 4.92 (d, 1H, J = 8.0 Hz, 5-CH), 5.20 (d, 1H, J = 6.4 Hz, 3′-CH), 5.58 (dd, J = 10.8, 7.2 Hz, 7-CH), 5.67 (d, 1H, J = 7.6 Hz, 2-CH), 6.22 (t, 1H, J = 9.2 Hz, 13-CH), 6.25 (s, 1H, 10-CH), 6.72 (d, 2H, J = 8.8 Hz, o-3″-PhOCH3), 7.23 (m, 5H, 3′-Ph), 7.41 (d, J = 9.2 Hz, m-3″-PhOCH3), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.03 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.66, 171.15, 170.16, 169.86, 166.82, 162.70, 153.21, 153.19, 142.51, 137.31, 133.87, 132.00, 132.19, 132.00, 130.05, 129.75, 129.01, 128.67, 128.55, 128.20, 127.93, 113.64, 100.84, 94.21, 94.19, 83.67, 81.98, 80.41, 79.02, 78.88, 74.24, 71.45, 65.28, 60.39, 56.08, 55.58, 49.23, 46.90, 43.07, 35.39, 33.86, 33.17, 28.89, 26.96, 26.23, 25.59, 24.90, 21.57, 21.04, 14.80, 14.20, 10.72. Anal. calcd. for C54H57Cl6NO19S: C, 51.12; H, 4.53; N, 1.10. Found: C, 51.33; H, 4.55; N, 1.14.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-isopropyl) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13e). Yield 87% (267 mg); mp 165–167 °C; 1H-NMR (CDCl3): δ 1.18 (s, 3H, 17-CH3), 1.22 (d, 3H, J = 1.6 Hz, 2″-CH3), 1.24 (d, 3H, J = 1.6 Hz, 2″-CH3), 1.26 (s, 3H, 16-CH3), 1.83 and 1.89 (2s, 6H, i-Pr), 1.96 (s, 3H, 19-CH3), 1.99 (s, 3H, 18-CH3), 2.00 (s, 3H, OAc), 2.15 (m, 2H, 14-CH2), 2.05 and 2.61 (2m, 2H, 6-CH2), 2.89 (m, 1H, 2″-CH), 3.89 (d, 1H, J = 7.2 Hz, 3-CH), 4.12 and 4.28 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.51 (d, 1H, J = 6.4 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (dd, 2H, J = 14.4, 12.0 Hz, Troc), 4.92 (d, 1H, J = 8.0 Hz, 5-CH), 5.24 (m, 1H, 3′-CH), 5.58 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.66 (d, 1H, J = 7.2 Hz, 2-CH), 6.22 (t, 1H, J = 9.2 Hz, 13-CH), 6.24 (s, 1H, 10-CH), 7.08 (d, 2H, J = 8.0 Hz, 3″-Ph), 7.14 (m, 2H, 3′-Ph), 7.20 (m, 3H, 3′-Ph), 7.39 (d, 2H, J = 8.8 Hz, 3″-Ph), 7.49 (t, 2H, J = 7.6 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.03 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.66, 171.15, 170.15, 169.88, 166.82, 153.91, 153.20, 153.19, 142.51, 137.01, 133.87, 131.99, 130.05, 129.01, 128.67, 128.47, 128.18, 127.97, 127.73, 126.52, 100.94, 94.22, 94.20, 83.67, 82.01, 80.41, 79.01, 78.88, 74.24, 71.46, 65.30, 60.39, 56.07, 53.43, 46.89, 43.07, 35.39, 34.09, 33.16, 28.82, 27.06, 26.23, 23.67, 23.58, 21.55, 21.03, 14.81, 14.20, 10.72. Anal. calcd. for C56H61Cl6NO18S: C, 52.51; H, 4.80; N, 1.09. Found: C, 52.75; H, 4.88; N, 1.15.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-fluoro) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13f). Yield 89% (268 mg); mp 166–168 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.84 and 1.90 (2s, 6H, i-Pr), 1.97 (s, 3H, 19-CH3), 2.01 (s, 3H, 18-CH3), 2.04 (s, 3H, OAc), 2.16 (m, 2H, 14-CH2), 2.05 and 2.62 (2m, 2H, 6-CH2), 3.90 (d, 1H, J = 6.8 Hz, 3-CH), 4.13 and 4.29 (2d, 2H, J = 8.8 Hz, 20-CH2), 4.52 (d, 1H, J = 6.4 Hz, 2′-CH), 4.62 and 4.94 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (dd, 2H, J = 13.0, 11.8 Hz, Troc), 4.93 (d, 1H, J = 9.2 Hz, 5-CH), 5.25 (d, 1H, J = 6.4 Hz, 3′-CH), 5.59 (dd, 1H, J = 10.8, 7.2 Hz, 5-CH), 5.67 (d, 1H, J = 7.2 Hz, 2-CH), 6.24 (t, 1H, J = 8.4 Hz, 13-CH), 6.25 (s, 1H, 10-CH), 6.90 (t, 2H, J = 8.4 Hz, -PhF), 7.20 (m, 4H, 3′-Ph), 7.26 (m, 1H, 3′-Ph), 7.45 (m, 2H, -PhF), 7.49 (t, 2H, J = 7.6 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.03 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 171.17, 170.13, 169.77, 166.84, 166.02, 163.49, 153.21, 142.42, 136.71, 133.90, 132.05, 130.30, 130.20, 130.05, 128.97, 128.68, 128.65, 128.46, 128.11, 115.70, 115.47, 101.12, 94.18, 83.68, 82.03, 80.44, 79.00, 78.89, 74.21, 71.55, 65.15, 60.40, 56.08, 46.91, 43.07, 35.36, 33.17, 28.59, 27.26, 26.22, 21.59, 21.06, 21.02, 14.85, 14.21, 10.73. Anal. calcd. for C53H54Cl6FNO18S: C, 50.65; H, 4.33; N, 1.11. Found: C, 50.82; H, 4.37; N, 1.18.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-trifluoro methyl) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13g). Yield 100% (312 mg); mp 165–167 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.84 and 1.92 (2s, 6H, i-Pr), 1.99 (s, 3H, 19-CH3), 2.02 (s, 3H, 18-CH3), 2.03 (s, 3H, OAc), 2.16 (m, 2H, 14-CH2), 2.05 and 2.62 (2m, 2H, 6-CH2), 3.90 (d, 1H, J = 6.8 Hz, 3-CH), 4.13 and 4.29 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.54 (d, 1H, J = 6.4 Hz, 2′-CH), 4.63 and 4.94 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (dd, 2H, J = 13.6, 12.0 Hz, Troc), 4.93 (d, 1H, J = 7.6 Hz, 5-CH), 5.29 (dd, 1H, J = 6.0 Hz, 3′-CH), 5.59 (dd, 1H, J = 10.8, 6.8 Hz, 7-CH), 5.67 (d, 1H, J = 6.8 Hz, 2-CH), 6.24 (t, 1H, J = 9.2 Hz, 13-CH), 6.26 (s, 1H, 10-CH), 7.14 (m, 1H, 3′-Ph), 7.23 (m, 4H, 3′-Ph), 7.47 (d, 2H, J = 8.4 Hz, -PhCF3), 7.53 (d, 2H, J = 8.4 Hz, -PhCF3), 7.49 (t, 2H, J = 8.0 Hz, m-OBz), 7.37 (t, 1H, J = 7.6 Hz, p-OBz), 8.02 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.64, 171.17, 170.10, 169.66, 166.84, 153.21, 144.07, 142.32, 136.19, 134.04, 133.90, 133.71, 132.11, 130.05, 128.96, 128.68, 128.60, 127.94, 125.49, 125.45, 124.47, 121.76, 101.32, 94.18, 83.67, 82.00, 80.46, 78.98, 74.20, 71.62, 65.16, 60.40, 56.08, 46.92, 43.08, 35.36, 33.17, 28.42, 27.55, 26.22, 21.60, 21.06, 21.00, 14.85, 14.20, 10.72. Anal. calcd. for C54H54Cl6F3NO18S: C, 49.63; H, 4.17; N, 1.07. Found: C, 49.85; H, 4.22; N, 1.13.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-chloro) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13h). Yield 91% (277 mg); mp 169–171 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.84 and 1.89 (2s, 6H, i-Pr), 1.96 (s, 3H, 19-CH3), 2.01 (s, 3H, 18-CH3), 2.03 (s, 3H, OAc), 2.16 (m, 2H, 14-CH2), 2.05 and 2.61 (2m, 2H, 6-CH2), 3.90 (d, 1H, J = 7.2 Hz, 3-CH), 413 and 4.29 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.53 (d, 1H, J = 6.4 Hz, 2′-CH), 4.62 and 4.94 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (dd, 2H, J = 13.6, 11.6 Hz, Troc), 4.93 (d, 1H, J = 8.0 Hz, 5-CH), 5.25 (d, 1H, J = 6.4 Hz, 3′-CH), 5.58 (dd, 1H, J = 10.8, 7.2 Hz, 2-CH), 5.67 (d, 1H, J = 7.2 Hz, 2-CH), 6.24 (t, 1H, J = 9.2 Hz, 13-CH), 6.25 (s, 1H, 10-CH), 7.20 (d, 2H, J = 9.2 Hz, -PhCl), 7.20 (m, 4H, 3′-Ph), 7.28 (m, 1H, 3′-Ph), 7.36 (d, 2H, J = 8.8 Hz, -PhCl), 7.49 (t, 2H, J = 7.6 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.03 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 171.18, 170.13, 169.73, 166.84, 153.21, 142.40, 139.11, 138.95, 136.66, 133.90, 138.95, 136.66, 133.90, 132.06, 130.05, 128.97, 128.92, 128.68, 128.46, 128.14, 101.10, 94.20, 94.18, 83.67, 81.98, 80.44, 78.99, 78.89, 74.21, 71.55, 65.18, 60.41, 56.08, 46.91, 43.07, 35.37, 33.17, 28.58, 27.31, 26.23, 21.60, 21.06, 21.02, 14.84, 14.21, 10.73. Anal. calcd. for C53H54Cl7NO18S: C, 50.00; H, 4.27; N, 1.10. Found: C, 50.23; H, 4.34; N, 1.15.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(4-bromo) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13i). Yield 98% (309 mg); mp 166–168 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.84 and 1.89 (2s, 6H, i-Pr), 1.96 (s, 3H, 19-CH3), 2.01 (s, 3H, 18-CH3), 2.03 (s, 3H, OAc), 2.16 (m, 2H, 14-CH2), 2.06 and 2.62 (2m, 2H, 6-CH2), 3.90 (d, 1H, J = 6.8 Hz, 3-CH), 4.13 and 4.29 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.53 (d, 1H, J = 6.0 Hz, 2′-CH), 4.62 and 4.94 (2d, 2H, J = 11.8 Hz, Troc), 4.80 (dd, 2H, J = 13.8, 11.8 Hz, Troc), 4.93 (d, 1H, J = 8.0 Hz, 5-CH), 5.25 (d, 1H, J = 6.0 Hz, 3′-CH), 5.59 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.67 (d, 1H, J = 7.2 Hz, 2-CH), 6.24 (t, 1H, J = 9.2 Hz, 13-CH), 6.25 (s, 1H, 10-CH), 7.20 (m, 4H, 3′-Ph), 7.28 (m, 1H, 3′-Ph), 7.29 (d, 2H, J = 8.8 Hz, -PhBr), 7.37 (d, 2H, J = 8.8 Hz, -PhBr), 7.50 (t, 2H, J = 7.8 Hz, m-OBz), 7.64 (t, 1H, J = 7.4 Hz, p-OBz), 8.03 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 170.12, 169.73, 166.85, 153.21, 142.39, 139.64, 136.63, 133.90, 132.06, 131.66, 130.06, 129.00, 128.96, 128.71, 128.46, 128.13, 127.49, 101.10, 94.18, 83.67, 81.97, 80.44, 78.99, 78.90, 74.20, 71.56, 65.20, 60.41, 56.08, 46.91, 43.08, 35.36, 33.17, 28.58, 27.32, 26.23, 21.60, 21.01, 14.84, 14.21, 10.73. Anal. calcd. for C53H54BrCl6NO18S: C, 48.31; H, 4.13; N, 1.06. Found: C, 48.53; H, 4.23; N, 1.14.
7,10-Di(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III-13-O-[2,2-dimethyl-3-(2,4,6-trimethyl) benzene sulfonyl-4-phenyl-oxazolidine-5-carboxylate] (13j). Yield 81% (248 mg); mp 167–169 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.28 (s, 3H, 16-CH3), 1.84 and 1.96 (2s, 6H, i-Pr), 1.98 (s, 3H, 19-CH3), 2.03 (s, 3H, 18-CH3), 2.08 (s, 3H, OAc), 2.12 (s, 3H, p-3″-CH3), 2.14 (m, 2H, 14-CH2), 2.05 and 2.62 (2m, 2H, 6-CH2), 2.54 (s, 6H, o-3″-CH3), 3.91 (d, 1H, J = 7.2 Hz, 3-CH), 4.13 and 4.28 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.44 (d, 1H, J = 6.8 Hz, 2′-CH), 4.63 and 4.94 (2d, 2H, J = 11.8 Hz, Troc), 4.80 (s, 2H, Troc), 4.93 (d, 1H, J = 8.0 Hz, 5-CH), 5.15 (d, 1H, J = 6.8 Hz, 3′-CH), 5.59 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.67 (d, 1H, J = 7.2 Hz, 2-CH), 6.27 (t, 1H, J = 8.2 Hz, 13-CH), 6.27 (s, 1H, 10-CH), 6.55 (s, 2H, 3″-Ph), 6.95–7.09 (m, 5H, 3′-Ph), 7.49 (t, 2H, J = 7.8 Hz, m-OBz), 7.63 (t, 1H, J = 7.6 Hz, p-OBz), 8.02 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.72, 171.18, 170.17, 170.09, 166.83, 153.21, 153.19, 143.12, 142.62, 140.07, 136.70, 133.85, 132.93, 131.91, 131.69, 130.07, 129.00, 128.65, 128.00, 127.66, 126.91, 102.19, 94.20, 83.70, 82.46, 80.38, 79.02, 78.94, 74.23, 71.45, 65.32, 60.41, 56.06, 46.90, 43.06, 35.38, 33.17, 29.70, 29.05, 26.89, 26.33, 23.04, 21.53, 21.04, 20.72, 14.90, 14.21, 10.73. Anal. calcd. for C56H61Cl6NO18S: C, 52.51; H, 4.80; N, 1.09. Found: C, 52.75; H, 4.88; N, 1.17.
3.2.5. General Procedure for the Synthesis of 5a–j
To a round-bottomed flask (25 mL) were added 13a–j (0.218 mmol) and HCOOH (>98%, 5 mL). The reaction mixture was stirred at room temperature for 4 h. Then the resulting solution was neutralized by addition of saturated NaHCO3. After extracted with EtOAc three times, the combined organic phase was washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The obtained residue was purified by silica gel flash chromatography column (acetone/petroleum ether: 1/3) to afford 5a–j as white solids.
N-De-tert-butoxycarbonyl-N-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5a). Yield 72% (188 mg); mp 156–158 °C; 1H-NMR (CDCl3): δ 1.19 (s, 3H, 17-CH3), 1.25 (s, 3H, 16-CH3), 1.86 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.25 (m, 2H, 14-CH2), 2.34 (s, 3H, OAc), 2.06 and 2.61 (2m, 2H, 6-CH2), 3.86 (d, 1H, J = 6.8 Hz, 3-CH), 4.20 and 4.31 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.54 (d, 1H, J = 3.2 Hz, 2′-CH), 4.60 and 4.91 (2d, 2H, J = 12.0 Hz, Troc), 4.78 (s, 2H, Troc), 4.93 (d, 1H, J = 9.6 Hz, 5-CH), 4.93 (m, 1H, 3′-CH), 5.52 (m, 1H, 7-CH), 5.67 (d, 1H, J = 6.8 Hz, 2-CH), 5.79 (m, 1H, -CONH-), 6.16 (t, 1H, J = 8.8 Hz, 13-CH), 6.21 (s, 1H, 10-CH), 7.09 (m, 2H, 3′-Ph), 7.18 (m, 3H, 3′-Ph), 7.28 (t, 2H, J = 8.0 Hz, m-PhSO2), 7.42 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.48 (t, 2H, J = 7.6 Hz, m-OBz), 7.61 (t, 1H, J = 7.6 Hz, p-OBz), 7.62 (d, 2H, J = 7.6 Hz, o-PhSO2), 8.08 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.62, 170.61, 166.78, 153.22, 142.02, 140.18, 136.31, 133.88, 132.62, 132.30, 130.13, 129.04, 128.81, 128.72, 128.65, 128.36, 127.05, 126.91, 94.17, 83.60, 80.95, 79.12, 78.61, 74.62, 74.12, 72.10, 60.42, 59.50, 56.27, 46.92, 43.02, 35.53, 33.28, 26.50, 22.46, 20.73, 14.91, 14.20, 10.73. Anal. calcd. for C50H51Cl6NO18S: C, 50.10; H, 4.29; N, 1.17. Found: C, 50.33; H, 4.43; N, 1.23.
N-De-tert-butoxycarbonyl-N-phenylmethylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5b). Yield 79% (208 mg); mp 149–151 °C; 1H-NMR (CDCl3): δ 1.20 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.76 (s, 3H, 19-CH3), 1.91 (s, 3H, 18-CH3), 2.29 (m, 2H, 14-CH2), 2.35 (s, 3H, OAc), 2.07 and 2.62 (2m, 2H, 6-CH2), 3.89 (d, 1H, J = 7.2 Hz, 3-CH), 4.04 (s, 2H, 3″-CH2), 4.22 and 4.32 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.54 (br s, 1H, 2′-CH), 4.61 and 4.92 (2d, 2H, J = 11.6 Hz, Troc), 4.79 (s, 2H, Troc), 4.88 (m, 1H, 3′-CH), 4.95 (d, 1H, J = 9.2 Hz, 5-CH), 5.53 (dd, 1H, J = 10.8, 7.2 Hz, 2-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 6.22 (s, 1H, 10-CH), 6.25 (t, 1H, J = 8.0 Hz, 13-CH), 7.07 (d, 2H, J = 7.2 Hz, 3″-Ph), 7.22 (t, 2H, J = 7.2 Hz, 3′-Ph), 7.35 (d, 2H, J = 7.6 Hz, 3″-Ph), 7.42 (m, 3H, 3′-Ph), 7.48 (t, 2H, J = 8.0 Hz, m-OBz), 7.63 (t, 1H, J = 7.4 Hz, p-OBz), 8.10 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.65, 171.53, 170.52, 166.75, 153.22, 153.17, 142.17, 137.52, 133.82, 132.20, 130.71, 130.16, 129.08, 129.02, 128.83, 128.68, 128.59, 128.44, 127.48, 94.18, 83.62, 80.89, 79.12, 78.61, 74.68, 74.19, 72.15, 60.41, 60.25, 59.57, 58.47, 56.24, 46.87, 43.04, 35.54, 33.81, 33.26, 29.69, 26.45, 25.57, 24.89, 22.47, 21.05, 20.84, 18.40, 14.79, 14.20, 10.73. Anal. calcd. for C51H53Cl6NO18S: C, 50.51; H, 4.40; N, 1.15. Found: C, 50.73; H, 4.53; N, 1.16.
N-De-tert-butoxycarbonyl-N-(4-methyl)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5c). Yield 85% (224 mg); mp 157–159 °C; 1H-NMR (CDCl3): δ 1.20 (s, 3H, 17-CH3), 1.26 (s, 3H, 16-CH3), 1.88 (s, 3H, 19-CH3), 1.91 (s, 3H, 18-CH3), 2.26 (m, 2H, 14-CH2), 2.34 (s, 3H, OAc), 2.35 (s, 3H, CH3 in 4-methylphenyl), 2.08 and 2.63 (2m, 2H, 6-CH2), 3.88 (d, 1H, J = 6.8 Hz, 3-CH), 4.22 and 4.32 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.56 (d, 1H, J = 3.2 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (s, 2H, Troc), 4.91 (m, 1H, 3′-CH), 4.94 (d, 1H, J = 9.2 Hz, 5-CH), 5.54 (m, 1H, 7-CH), 5.69 (d, 1H, J = 6.8 Hz, 2-CH), 5.84 (m, 1H, -CONH-), 6.18 (t, 1H, J = 9.0 Hz, 13-CH), 6.22 (s, 1H, 10-CH), 7.08 (d, 2H, J = 8.4 Hz, m-PhSO2), 7.13 (m, 2H, 3′-Ph), 7.21 (m, 3H, 3′-Ph), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.53 (d, 2H, J = 8.4 Hz, o-PhSO2), 7.64 (t, 1H, J = 7.4 Hz, p-OBz), 8.10 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.64, 170.58, 166.78, 153.22, 153.20, 143.51, 142.10, 137.25, 136.58, 133.87, 132.22, 130.14, 129.40, 129.07, 128.72, 128.62, 128.24, 127.08, 126.96, 94.17, 83.61, 80.90, 79.11, 78.63, 74.60, 74.17, 72.06, 59.51, 56.24, 46.90, 43.02, 35.50, 26.44, 22.46, 21.45, 20.79, 14.85, 10.73. Anal. calcd. for C51H53Cl6NO18S: C, 50.51; H, 4.40; N, 1.15. Found: C, 50.75; H, 4.51; N, 1.19.
N-De-tert-butoxycarbonyl-N-(4-methoxyl)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5d). Yield 79% (210 mg); mp 152–154 °C; 1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.86 (s, 3H, 19-CH3), 1.92 (s, 3H, 18-CH3), 2.22 (m, 2H, 14-CH2), 2.36 (s, 3H, OAc), 2.08 and 2.64 (2m, 2H, 6-CH2), 3.80 (s, 3H, OCH3), 3.89 (d, 1H, J = 7.2 Hz, 3-CH), 4.22 and 4.34 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.55 (d, 1H, J = 3.2 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (s, 2H, Troc), 4.91 (m, 1H, 3′-CH), 4.96 (d, 1H, J = 9.2 Hz, 5-CH), 5.54 (dd, J = 10.8, 7.2 Hz, 7-CH), 5.69 (d, 1H, J = 6.8 Hz, 2-CH), 5.74 (m, 1H, -CONH-), 6.16 (t, 1H, J = 8.8 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 6.76 (m, 2H, o-PhOCH3), 7.15 (m, 2H, 3′-Ph), 7.23 (m, 3H, 3′-Ph), 7.51 (t, 2H, J = 7.6 Hz, m-OBz), 7.58 (d, 2H, J = 8.8 Hz, m-PhOCH3), 7.64 (t, 1H, J = 7.2 Hz, p-OBz), 8.10 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.64, 171.18, 170.57, 166.77, 162.83, 153.22, 136.72, 133.87, 132.22, 131.87, 130.13, 129.12, 128.72, 128.66, 128.27, 127.09, 113.94, 94.18, 83.60, 80.92, 79.12, 78.62, 74.13, 72.00, 60.40, 56.27, 55.57, 46.91, 43.02, 35.53, 33.80, 33.29, 26.43, 25.56, 24.88, 22.44, 21.05, 20.74, 14.90, 14.20, 10.72. Anal. calcd. for C51H53Cl6NO19S: C, 49.85; H, 4.35; N, 1.14. Found: C, 49.99, H, 4.43, N, 1.21.
N-De-tert-butoxycarbonyl-N-(4-isopropyl)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5e). Yield 77% (208 mg); mp 165–167 °C; 1H-NMR (CDCl3): δ 1.20 (br s, 6H, 2CH3 in i-Pr), 1.21 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.88 (s, 3H, 19-CH3), 1.92 (s, 3H, 18-CH3), 2.29 (m, 2H, 14-CH2), 2.37 (s, 3H, OAc), 2.08 and 2.64 (2m, 2H, 6-CH2), 2.88 (m, 1H, CH in i-Pr), 3.89 (d, 1H, J = 6.4 Hz, 3-CH), 4.23 and 4.32 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.56 (d, 1H, J = 3.6 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (s, 2H, Troc), 4.93 (m, 1H, 3′-CH), 4.94 (d, 1H, J = 9.2 Hz, 5-CH), 5.54 (dd, 1H, J = 10.6, 7.4 Hz, 7-CH), 5.70 (d, 1H, J = 6.8 Hz, 2-CH), 6.23 (s, 1H, 10-CH), 6.23 (t, 1H, J = 9.0 Hz, 13-CH), 7.01-7.15 (m, 8H, 3′-Ph and i-PrPh), 7.52 (m, 2H, i-PrPh), 7.52 (t, 2H, J = 7.6 Hz, m-OBz), 7.63 (t, 1H, J = 7.2 Hz, p-OBz), 8.11 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (100 MHz, CDCl3) δ 200.65, 171.23, 170.63, 166.74, 154.17, 153.22, 142.15, 137.36, 136.21, 133.82, 132.22, 130.15, 129.13, 128.71, 128.51, 128.21, 127.09, 126.84, 94.18, 83.62, 80.88, 79.12, 78.62, 74.66, 74.21, 72.06, 60.41, 59.63, 56.22, 46.91, 43.02, 35.62, 34.08, 33.27, 26.45, 23.65, 23.60, 22.47, 21.05, 20.87, 14.83, 14.20, 10.75. Anal. calcd. for C53H57Cl6NO18S: C, 51.30; H, 4.63; N, 1.13. Found: C, 51.53; H, 4.72; N, 1.18.
N-De-tert-butoxycarbonyl-N-(4-fluoro)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5f). Yield 50% (132 mg); mp 162–164 °C;1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.26 (s, 3H, 16-CH3), 1.88 (s, 3H, 19-CH3), 1.92 (s, 3H, 18-CH3), 2.27 (m, 2H, 14-CH2), 2.36 (s, 3H, OAc), 2.08 and 2.63 (2m, 2H, 6-CH2), 3.88 (d, 1H, J = 6.8 Hz, 3-CH), 4.22 and 4.33 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.56 (d, 1H, J = 3.6 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (s, 2H, Troc), 4.95 (d, 1H, J = 9.6 Hz, 3′-CH), 4.96 (d, 1H, J = 9.2 Hz, 5-CH), 5.53 (dd, 1H, J = 10.6, 7.4 Hz, 5-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 5.92 (d, 1H, J = 9.2 Hz, -CONH-), 6.20 (t, 1H, J = 8.0 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 6.94 (t, 2H, J = 8.4 Hz, -PhF), 7.11 (m, 2H, 3′-Ph), 7.21 (m, 3H, 3′-Ph), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.62 (m, 2H, -PhF), 7.63 (t, 1H, J = 7.6 Hz, p-OBz), 8.10 (d, 2H, J = 8.0 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.58, 171.21, 171.08, 170.66, 166.77, 166.16, 163.63, 153.22, 141.91, 136.30, 136.12, 133.90, 132.40, 130.12, 129.71, 129.62, 129.02, 128.71, 128.69, 128.47, 127.13, 126.89, 116.05, 115.82, 94.16, 83.58, 81.01, 79.10, 78.58, 74.62, 74.10, 72.11, 60.42, 59.57, 58.48, 56.29, 46.94, 43.02, 35.51, 33.28, 26.47, 22.45, 21.05, 20.67, 18.41, 14.94, 14.20, 10.72. Anal. calcd. for C50H50Cl6FNO18S: C, 49.36; H, 4.14; N, 1.15. Found: C, 49.58; H, 4.22; N, 1.16.
N-De-tert-butoxycarbonyl-N-(4-trifluoromethyl)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxy carbonyl)-docetaxel (5g). Yield 56% (154 mg); mp 159–161 °C; 1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.89 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.29 (m, 2H, 14-CH2), 2.36 (s, 3H, OAc), 2.09 and 2.64 (2m, 2H, 6-CH2), 3.88 (d, 1H, J = 6.8 Hz, 3-CH), 4.23 and 4.33 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.58 (d, 1H, J = 2.4 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (s, 2H, Troc), 4.96 (d, 1H, J = 9.6 Hz, 5-CH), 5.01 (dd, 1H, J = 9.2, 7.2 Hz, 3′-CH), 5.53 (dd, 1H, J = 10.6, 7.4 Hz, 7-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 6.00 (d, 1H, J = 9.6 Hz, -CONH-), 6.22 (t, 1H, J = 8.2 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 7.07 (m, 2H, 3′-Ph), 7.17 (m, 3H, 3′-Ph), 7.50 (d, 2H, J = 8.4 Hz, -PhCF3), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 7.70 (d, 2H, J = 8.4 Hz, -PhCF3), 8.09 (d, 2H, J = 7.2 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.54, 170.86, 170.76, 166.78, 153.24, 143.76, 141.74, 135.70, 133.93, 132.53, 130.11, 128.98, 128.72, 128.68, 128.54, 127.41, 127.17, 125.79, 94.15, 83.55, 81.10, 79.10, 78.54, 74.57, 74.03, 72.12, 59.60, 58.49, 56.33, 46.97, 43.02, 35.50, 33.30, 26.52, 22.46, 21.05, 20.56, 18.42, 15.00, 14.20, 10.71. Anal. calcd. for C51H50Cl6F3NO18S: C, 48.36; H, 3.98; N, 1.11. Found: C, 48.59; H, 4.11; N, 1.15.
N-De-tert-butoxycarbonyl-N-(4-chloro)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5h). Yield 67% (180 mg); mp 162–164 °C; 1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.88 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.27 (m, 2H, 14-CH2), 2.35 (s, 3H, OAc), 2.08 and 2.64 (2m, 2H, 6-CH2), 3.88 (d, 1H, J = 6.8 Hz, 3-CH), 4.22 and 4.33 (2d, 2H, J = 8.8 Hz, 20-CH2), 4.57 (br s, 1H, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 12.0 Hz, Troc), 4.80 (s, 2H, Troc), 4.96 (d, 1H, J = 9.6 Hz, 5-CH), 4.97 (d, 1H, J = 9.2 Hz, 3′-CH), 5.53 (dd, 1H, J = 10.4, 7.0 Hz, 2-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 5.92 (d, 1H, J = 9.2 Hz, -CONH-), 6.19 (t, 1H, J = 8.8 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 7.12 (m, 2H, -PhCl), 7.23 (m, 5H, 3′-Ph), 7.50 (t, 2H, J = 7.6 Hz, m-OBz), 7.53 (d, 2H, J = 8.8 Hz, -PhCl), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.09 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.57, 171.01, 170.68, 166.77, 153.22, 141.85, 139.09, 138.78, 136.12, 133.92, 132.43, 130.12, 129.00, 128.72, 128.48, 128.36, 127.14, 94.17, 83.57, 81.03, 79.10, 78.57, 74.58, 74.07, 72.12, 60.42, 59.55, 56.30, 46.95, 43.02, 35.51, 33.29, 26.49, 22.46, 20.64, 14.95, 14.20, 10.72. Anal. calcd. for C50H50Cl7NO18S: C, 48.70; H, 4.09; N, 1.14. Found: C, 48.95; H, 4.11; N, 1.18.
N-De-tert-butoxycarbonyl-N-(4-bromo)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxycarbonyl)-docetaxel (5i). Yield 57% (158 mg); mp 155–157 °C; 1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.27 (s, 3H, 16-CH3), 1.88 (s, 3H, 19-CH3), 1.91 (s, 3H, 18-CH3), 2.27 (m, 2H, 14-CH2), 2.35 (s, 3H, OAc), 2.08 and 2.63 (2m, 2H, 6-CH2), 3.88 (d, 1H, J = 7.2 Hz, 3-CH), 4.22 and 4.33 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.60 (t, 1H, J = 3.6 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.80 (s, 2H, Troc), 4.96 (d, 1H, J = 9.6 Hz, 5-CH), 4.97 (d, 1H, J = 9.2 Hz, 3′-CH), 5.53 (dd, 1H, J = 10.8, 7.2 Hz, 7-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 5.92 (d, 1H, J = 9.2 Hz, -CONH-), 6.20 (t, 1H, J = 8.8 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 7.11 (m, 2H, 3′-Ph), 7.24 (m, 3H, 3′-Ph), 7.40 (m, 2H, -PhBr), 7.46 (m, 2H, -PhBr), 7.50 (t, 2H, J = 7.8 Hz, m-OBz), 7.64 (t, 1H, J = 7.6 Hz, p-OBz), 8.09 (d, 2H, J = 7.6 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.57, 171.21, 171.02, 170.67, 166.77, 153.22, 141.85, 139.33, 136.12, 133.91, 132.43, 131.98, 130.12, 129.01, 128.72, 128.43, 127.55, 127.15, 126.96, 94.16, 83.57, 81.03, 79.10, 78.57, 74.57, 74.08, 72.11, 60.42, 59.57, 58.48, 56.30, 53.43, 46.95, 43.03, 35.52, 33.29, 26.51, 22.46, 21.05, 20.64, 18.42, 14.95, 14.20, 10.72. Anal. calcd. for C50H50BrCl6NO18S: C, 47.00; H, 3.94; N, 1.10. Found: C, 47.30; H, 4.03; N, 1.17.
N-De-tert-butoxycarbonyl-N-(2,4,6-trimethyl)-phenylsulfonyl 7,10-di(2,2,2-trichloroethyloxy carbonyl)-docetaxel (5j). Yield 68% (183 mg); mp 151–153 °C; 1H-NMR (CDCl3): δ 1.21 (s, 3H, 17-CH3), 1.26 (s, 3H, 16-CH3), 1.87 (s, 3H, 19-CH3), 1.92 (s, 3H, 18-CH3), 2.29 (m, 2H, 14-CH2), 2.23 (s, 3H, p-3″-CH3), 2.32 (s, 3H, OAc), 2.08 and 2.63 (2m, 2H, 6-CH2), 2.50 (s, 6H, o-3″-CH3), 3.88 (d, 1H, J = 6.8 Hz, 3-CH), 4.21 and 4.33 (2d, 2H, J = 8.6 Hz, 20-CH2), 4.54 (d, 1H, J = 3.6 Hz, 2′-CH), 4.62 and 4.93 (2d, 2H, J = 11.6 Hz, Troc), 4.75 (m, 1H, 3′-CH), 4.80 (s, 2H, Troc), 4.96 (d, 1H, J = 9.6 Hz, 5-CH), 5.53 (dd, 1H, J = 10.4, 7.2 Hz, 7-CH), 5.66 (d, 1H, J = 8.8 Hz, -CONH-), 5.70 (d, 1H, J = 6.8 Hz, 2-CH), 6.12 (t, 1H, J = 8.8 Hz, 13-CH), 6.23 (s, 1H, 10-CH), 6.78 (s, 2H, 3″-Ph), 7.10-7.22 (m, 5H, 3′-Ph), 7.53 (t, 2H, J = 7.8 Hz, m-OBz), 7.67 (t, 1H, J = 7.2 Hz, p-OBz), 8.10 (d, 2H, J = 7.2 Hz, o-OBz). 13C-NMR (CDCl3) δ 200.63, 171.51, 170.31, 166.77, 153.22, 153.18, 142.38, 142.09, 138.60, 136.74, 134.20, 133.90, 132.26, 131.83, 130.08, 129.05, 128.71, 128.54, 128.35, 126.94, 94.17, 83.61, 80.90, 79.11, 78.59, 74.34, 74.10, 72.24, 59.39, 58.48, 56.24, 46.86, 43.05, 35.26, 33.27, 31.92, 29.68, 26.43, 22.87, 22.48, 20.85, 18.42, 14.73, 10.71. Anal. calcd. for C53H57Cl6NO18S: C, 51.30; H, 4.63; N, 1.13. Found: C, 51.53; H, 4.65; N, 1.17.
3.2.6. General Procedure for the Synthesis of 3a–j
To a solution of 5a–j (0.19 mmol) in methanol (10 mL) were added glacial acetic acid (4.60 mL) and zinc powder (0.46 g, 7.08 mmol). After stirred at 50 °C for 1 h, the reaction mixture was filtered to remove the zinc and solid formed. The filtrate was evaporated by distillation to give a white solid. The obtained solid was then dissolved in ethyl acetate (60 mL), which was washed with saturated NaHCO3, brine, dried over Na2SO4, and concentrated in vacuo. The obtained residue was further purified by silica gel flash chromatography column (petroleum ether/acetone: 2/1) to give 3a–j.
N-De-tert-butoxycarbonyl-N-phenylsulfonyl docetaxel (3a). White powder; Yield 75% (121 mg); mp 174–175 °C; 1H-NMR (CDCl3): δ 1.09 (s, 3H, 17-CH3), 1.19 (s, 3H, 16-CH3), 1.73 (s, 3H, 19-CH3), 1.81 (s, 3H, 18-CH3), 2.15 (m, 2H, 14-CH2), 2.30 (s, 3H, OAc), 1.82 and 2.51 (2m, 2H, 6-CH2), 3.84 (d, 1H, J = 7.2 Hz, 3-CH), 4.20 and 4.26 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.23 (m, 1H, 7-CH), 4.50 (d, 1H, J = 4.0 Hz, 2′-CH), 4.89 (d, 1H, J = 9.6 Hz, 5-CH), 4.89 (m, 1H, 3′-CH), 5.22 (s, 1H, 10-CH), 5.63 (d, 1H, J = 7.2 Hz, 2-CH), 6.14 (t, 1H, J = 8.8 Hz, 13-CH), 6.35 (d, 1H, J = 6.0 Hz, -CONH-), 7.09 (m, 2H, 3′-Ph), 7.14 (m, 3H, 3′-Ph), 7.24 (t, 2H, J = 8.0 Hz, m-PhSO2), 7.40 (t, 1H, J = 7.6 Hz, p-PhSO2), 7.46 (t, 2H, J = 7.6 Hz, m-OBz), 7.58 (t, 1H, J = 7.6 Hz, p-OBz), 7.59 (d, 2H, J = 7.6 Hz, o-PhSO2), 8.07 (d, 2H, J = 7.6 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.54, 171.85, 170.02, 165.73, 141.70, 137.67, 137.40, 136.71, 133.15, 131.98, 130.50, 130.00, 128.56, 128.48, 128.03, 127.57, 127.54, 126.77, 84.16, 80.86, 77.87, 77.76, 75.97, 75.23, 74.92, 74.28, 71.52, 71.26, 60.51, 57.63, 54.08, 46.52, 46.52, 43.25, 36.68, 36.10, 26.27, 22.17, 20.62, 13.55, 9.52; HRMS (ESI) m/z calcd. for C44H49NO14SNa+ [M+Na+]: 870.2771, found 870.2802.
N-De-tert-butoxycarbonyl-N-phenylmethylsulfonyl docetaxel (3b). White powder; Yield 74% (121 mg); mp 168–170 °C; 1H-NMR (CDCOCD3): δ 1.14 (s, 3H, 17-CH3), 1.21 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.89 (s, 3H, 18-CH3), 2.17 (m, 2H, 14-CH2), 2.41 (s, 3H, OAc), 1.85 and 2.46 (2m, 2H, 6-CH2), 3.89 (s, 1H, 2′-OH), 3.92 (d, 1H, J = 7.2 Hz, 3-CH), 4.09 and 4.22 (2d, 2H, J = 14.0 Hz, CH2Ph), 4.16 and 4.19 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.32 (m, 1H, 7-CH), 4.36 (br s, 1H, 10-OH), 4.64 (t, 1H, J = 5.2 Hz, 2′-CH), 4.97 (d, 1H, J = 9.2 Hz, 5-CH), 5.00 (d, 1H, J = 5.6 Hz, 3′-CH), 5.12 (m, 1H, -CONH-), 5.24 (d, 1H, J = 2.0 Hz,10-CH), 5.67 (d, 1H, J = 7.6 Hz, 2-CH), 6.22 (t, 1H, J = 8.8 Hz, 13-CH), 7.25-7.36 (m, 6H, 3′-Ph and 3″-Ph), 7.47 (t, 2H, J = 7.2 Hz, m-OBz), 7.55 (m, 4H, 3″-Ph), 7.66 (t, 1H, J = 7.2 Hz, p-OBz), 8.10 (d, 2H, J = 7.2 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.54, 172.23, 170.07, 165.76, 138.92, 137.38, 136.73, 133.15, 130.94, 130.45, 130.05, 129.98, 128.55, 128.51, 128.19, 128.14, 128.00, 127.93, 84.18, 80.83, 77.83, 75.98, 75.20, 75.00, 74.28, 71.52, 71.16, 60.70, 59.68, 57.63, 54.09, 46.48, 43.24, 36.67, 35.98, 26.22, 22.29, 20.61, 18.00, 13.52, 9.52; HRMS (ESI) m/z calcd. for C45H51NO14SNa+ [M+Na+]: 884.2928, found 884.2944.
N-De-tert-butoxycarbonyl-N-(4-methyl)-phenylsulfonyl docetaxel (3c). White powder; Yield 80% (131 mg); mp 172–174 °C; 1H-NMR (CDCl3): δ 1.10 (s, 3H, 17-CH3), 1.19 (s, 3H, 16-CH3), 1.73 (s, 3H, 19-CH3), 1.78 (s, 3H, 18-CH3), 2.14 (m, 2H, 14-CH2), 2.30 (s, 3H, OAc), 2.30 (s, 3H, CH3 in 4-methylphenyl), 1.83 and 2.52 (2m, 2H, 6-CH2), 3.84 (d, 1H, J = 6.8 Hz, 3-CH), 4.20 and 4.27 (2d, 2H, J = 8.4 Hz, 20-CH2), 4.21 (m, 1H, 7-CH), 4.50 (d, 1H, J = 3.6 Hz, 2′-CH), 4.89 (d, 1H, J = 9.2 Hz, 5-CH), 4.89 (m, 1H, 3′-CH), 5.20 (s, 1H, 10-CH), 5.63 (d, 1H, J = 7.6 Hz, 2-CH), 6.12 (m, 1H,-CONH-), 6.13 (t, 1H, J = 9.2 Hz, 13-CH), 7.04 (d, 2H, J = 8.0 Hz, m-PhSO2), 7.11 (m, 2H, 3′-Ph), 7.16 (m, 3H, 3′-Ph), 7.47 (t, 2H, J = 8.0 Hz, m-OBz), 7.49 (d, 2H, J = 8.4 Hz, o-PhSO2), 7.59 (t, 1H, J = 7.6 Hz, p-OBz), 8.07 (d, 2H, J = 7.6 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.55, 171.86, 170.05, 165.75, 142.66, 138.85, 137.93, 137.44, 136.66, 133.17, 130.48, 130.00, 129.08, 128.92, 128.50, 128.20, 128.03, 127.79, 127.59, 127.43, 126.92, 126.84, 84.17, 80.86, 77.88, 77.77, 75.98, 75.23, 74.92, 74.29, 71.53, 71.26, 60.44, 57.64, 46.52, 43.24, 36.67, 36.09, 26.25, 22.16, 20.61, 20.47, 17.99, 13.55, 9.53; HRMS (ESI) m/z calcd. for C45H51NO14SNa+ [M+Na+]: 884.2928, found 884.2942.
N-De-tert-butoxycarbonyl-N-(4-methoxyl)-phenylsulfonyl docetaxel (3d). White powder; Yield 73% (122 mg); mp 172–174 °C; 1H-NMR (CDCOCD3): δ 1.16 (s, 3H, 17-CH3), 1.23 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.89 (s, 3H, 18-CH3), 2.25 (m, 2H, 14-CH2), 2.40 (s, 3H, OAc), 1.86 and 2.45 (2m, 2H, 6-CH2), 3.82 (s, 3H, OCH3), 3.93 (d, 1H, J = 6.8 Hz, 3-CH), 3.93 (s, 1H, 2′-OH), 4.16 and 4.22 (2d, 2H, J = 8.2 Hz, 20-CH2), 4.31 (m, 1H, 7-CH), 4.37 (br s, 1H, 10-OH), 4.56 (d, 1H, J = 4.0 Hz, 2′-CH), 4.91 (m, 1H, 3′-CH), 4.87 (m, 1H, -CONH-), 4.97 (d, 1H, J = 9.2 Hz, 5-CH), 5.24 (s, 1H, 10-CH), 5.69 (d, 1H, J = 6.8 Hz, 2-CH), 6.17 (t, 1H, J = 9.0 Hz, 13-CH), 6.85 (d, 2H, J = 8.8 Hz, o-PhOCH3), 7.16 (t, 1H, J = 7.2 Hz, 3′-Ph), 7.22 (t, 2H, J = 7.2 Hz, 3′-Ph), 7.28 (d, 1H, J = 7.2 Hz, 3′-Ph), 7.57 (t, 2H, J = 7.2 Hz, m-OBz), 7.60 (d, 2H, J = 8.8 Hz, m-PhOCH3), 7.67 (t, 1H, J = 7.2 Hz, p-OBz), 8.13 (d, 2H, J = 7.6 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.55, 171.88, 170.04, 165.75, 162.51, 137.88, 137.42, 136.69, 133.36, 133.16, 130.49, 130.01, 128.92, 128.50, 128.04, 127.61, 127.47, 113.69, 84.17, 80.85, 77.88, 75.97, 75.23, 74.95, 74.28, 71.53, 71.26, 60.44, 57.63, 55.12, 46.52, 43.24, 36.68, 36.11, 26.24, 22.17, 20.62, 13.57, 9.52; HRMS (ESI) m/z calcd. for C45H51NO15SNa+ [M+Na+]: 900.2877, found 900.2889.
N-De-tert-butoxycarbonyl-N-(4-isopropyl)-phenylsulfonyl docetaxel (3e). White powder; Yield 77% (130 mg); mp 171–173 °C; 1H-NMR (CDCOCD3): δ 1.17 (s, 3H, 17-CH3), 1.20 (s, 3H, 16-CH3), 1.22 (s, 3H, CH3 in i-Pr), 1.25 (s, 3H, CH3 in i-Pr), 1.75 (s, 3H, 19-CH3), 1.91 (s, 3H, 18-CH3), 2.29 (m, 2H, 14-CH2), 2.43 (s, 3H, OAc), 1.86 and 2.47 (2m, 2H, 6-CH2), 2.90 (m, 1H, CH in i-Pr), 3.95 (d, 1H, J = 7.2 Hz, 3-CH), 3.96 (s, 1H, 2′-OH), 4.17 and 4.24 (2d, 2H, J = 8.2 Hz, 20-CH2), 4.32 (m, 1H, 7-CH), 4.38 (br s, 1H, 10-OH), 4.57 (d, 1H, J = 4.8 Hz, 2′-CH), 4.93 (d, 1H, J = 4.4 Hz, 3′-CH), 4.98 (d, 1H, J = 9.2 Hz, 5-CH), 5.25 (s, 1H, 10-CH), 5.69 (d, 1H, J = 7.6 Hz, 2-CH), 6.25 (t, 1H, J = 9.0 Hz, 13-CH), 7.17 (m, 8H, 3′-Ph and i-PrPh), 7.55 (d, 2H, J = 8.0 Hz, i-PrPh), 7.56 (t, 2H, J = 7.6 Hz, m-OBz), 7.66 (t, 1H, J = 7.2 Hz, p-OBz), 8.14 (d, 2H, J = 7.2 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.56, 171.93, 170.08, 165.79, 153.27, 138.97, 137.49, 137.40, 136.69, 133.15, 130.50, 130.03, 128.49, 128.21, 127.96, 127.76, 127.64, 127.45, 127.06, 127.00, 126.51, 126.39, 84.19, 80.87, 77.91, 77.80, 75.99, 75.27, 74.91, 74.29, 71.52, 71.30, 60.48, 57.64, 46.51, 43.27, 36.67, 36.13, 33.84, 26.30, 23.13, 23.05, 22.21, 20.68, 17.99, 15.37, 13.58, 9.56; HRMS (ESI) m/z calcd. for C47H55NO14SNa+ [M+Na+]: 912.3241, found 912.3239.
N-De-tert-butoxycarbonyl-N-(4-fluoro)-phenylsulfonyl docetaxel (3f). White powder; Yield 53% (87 mg); mp 173–175 °C; 1H-NMR (CDCOCD3): δ 1.16 (s, 3H, 17-CH3), 1.23 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.24 (m, 2H, 14-CH2), 2.41 (s, 3H, OAc), 1.85 and 2.46 (2m, 2H, 6-CH2), 3.93 (d, 1H, J = 7.2 Hz, 3-CH), 3.93 (s, 1H, 2′-OH), 4.16 and 4.22 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.31 (m, 1H, 7-CH), 4.37 (br s, 1H, 10-OH), 4.57 (d, 1H, J = 4.8 Hz, 2′-CH), 4.95 (d, 1H, J = 4.8 Hz, 3′-CH), 4.97 (d, 1H, J = 9.0 Hz, 5-CH), 5.24 (s, 1H, 10-CH), 5.69 (d, 1H, J = 7.6 Hz, 2-CH), 6.19 (t, 1H, J = 8.8 Hz, 13-CH), 7.09 (t, 2H, J = 7.2 Hz, 3′-Ph), 7.18 (d, 1H, J = 7.2 Hz, 3′-Ph), 7.21 (d, 2H, J = 6.8 Hz, -PhF), 7.27 (d, 2H, J = 6.8 Hz, 3′-Ph), 7.57 (t, 2H, J = 8.0 Hz, m-OBz), 7.67 (t, 1H, J = 7.6 Hz, p-OBz), 7.71 (dd, 2H, J = 8.8, 5.2 Hz, -PhF), 8.12 (d, 2H, J = 8.0 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.53, 171.84, 170.05, 165.74, 165.71, 163.21, 138.03, 138.00, 137.45, 137.37, 136.74, 133.17, 130.48, 130.00, 129.79, 129.70, 128.49, 128.07, 127.68, 127.60, 115.59, 115.36, 84.17, 80.86, 77.87, 75.97, 75.23, 74.90, 74.27, 71.52, 71.23, 60.64, 57.63, 46.51, 43.25, 36.67, 36.12, 26.23, 22.18, 20.63, 13.56, 9.53; HRMS (ESI) m/z calcd. for C44H48FNO14SNa+ [M+Na+]: 888.2677, found 888.2700.
N-De-tert-butoxycarbonyl-N-(4-trifluoromethyl)-phenylsulfonyl docetaxel (3g). White powder; Yield 57% (99 mg); mp 168–170 °C; 1H-NMR (CDCOCD3): δ 1.16 (s, 3H, 17-CH3), 1.23 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.91 (s, 3H, 18-CH3), 2.24 (m, 2H, 14-CH2), 2.42 (s, 3H, OAc), 1.86 and 2.47 (2m, 2H, 6-CH2), 3.93 (s, 1H, 2′-OH), 3.93 (d, 1H, J = 6.0 Hz, 3-CH), 4.16 and 4.23 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.32 (m, 1H, 7-CH), 4.37 (br s, 1H, 10-OH), 4.59 (d, 1H, J = 4.8 Hz, 2′-CH), 4.97 (d, 1H, J = 8.8 Hz, 5-CH), 4.98 (d, 1H, J = 4.4 Hz, 3′-CH), 5.24 (s, 1H, 10-CH), 5.69 (d, 1H, J = 6.8 Hz, 2-CH), 6.23 (t, 1H, J = 9.0 Hz, 13-CH), 7.16 (m, 3H, 3′-Ph), 7.24 (d, 1H, J = 6.8 Hz, 3′-Ph), 7.56 (t, 2H, J = 7.6 Hz, m-OBz), 7.66 (t, 1H, J = 7.6 Hz, p-OBz), 7.66 (d, 2H, J = 8.0 Hz, -PhCF3), 7.84 (d, 2H, J = 8.0 Hz, -PhCF3), 8.12 (d, 2H, J = 8.0 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.51, 171.79, 170.06, 165.75, 145.38, 137.35, 137.03, 136.77, 133.16, 132.87, 132.55, 130.48, 130.00, 128.48, 128.06, 127.75, 127.66, 125.67, 125.63, 125.00, 122.30, 84.17, 80.87, 77.87, 75.97, 75.24, 74.82, 74.27, 71.52, 71.23, 60.81, 57.63, 46.51, 43.25, 36.66, 36.13, 26.24, 22.20, 20.65, 13.55, 9.53; HRMS (ESI) m/z calcd. for C45H48F3NO14SNa+ [M+Na+]: 938.2645, found 938.2651.
N-De-tert-butoxycarbonyl-N-(4-chloro)-phenylsulfonyl docetaxel (3h). White powder; Yield 65% (109 mg); mp 178–180 °C; 1H-NMR (CDCOCD3): δ 1.16 (s, 3H, 17-CH3), 1.23 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.24 (m, 2H, 14-CH2), 2.41 (s, 3H, OAc), 1.86 and 2.47 (2m, 2H, 6-CH2), 3.93 (d, 1H, J = 6.0 Hz, 3-CH), 3.93 (s, 1H, 2′-OH), 4.16 and 4.22 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.32 (m, 1H, 7-CH), 4.38 (br s, 1H, 10-OH), 4.58 (d, 1H, J = 4.8 Hz, 2′-CH), 4.95 (d, 1H, J = 4.8 Hz, 3′-CH), 4.97 (d, 1H, J = 10.2 Hz, 5-CH), 5.25 (s, 1H, 10-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 6.20 (t, 1H, J = 9.0 Hz, 13-CH), 7.21 (m, 3H, 3′-Ph), 7.28 (d, 1H, J = 6.8 Hz, 3′-Ph), 7.36 (d, 2H, J = 8.4 Hz, -PhCl), 7.57 (t, 2H, J = 7.6 Hz, m-OBz), 7.65 (d, 2H, J = 8.8 Hz, -PhCl), 7.67 (t, 1H, J = 7.2 Hz, p-OBz), 8.12 (d, 2H, J = 7.2 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.53, 171.83, 170.07, 165.76, 140.49, 137.60, 137.44, 137.39, 136.74, 133.19, 130.47, 130.00, 128.67, 128.62, 128.50, 128.09, 127.70, 127.60, 84.18, 80.86, 77.88, 75.98, 75.24, 74.86, 74.28, 71.53, 71.25, 60.67, 57.64, 46.51, 43.26, 36.66, 36.12, 26.27, 22.20, 20.65, 13.57, 9.55; HRMS (ESI) m/z calcd. for C44H48ClNO14SNa+ [M+Na+]: 904.2382, found 904.2359.
N-De-tert-butoxycarbonyl-N-(4-bromo)-phenylsulfonyl docetaxel (3i). White powder; Yield 61% (107 mg); mp 179–181 °C; 1H-NMR (CDCOCD3): δ 1.16 (s, 3H, 17-CH3), 1.24 (s, 3H, 16-CH3), 1.74 (s, 3H, 19-CH3), 1.90 (s, 3H, 18-CH3), 2.24 (m, 2H, 14-CH2), 2.41 (s, 3H, OAc), 1.86 and 2.46 (2m, 2H, 6-CH2), 3.93 (s, 1H, 2′-OH), 3.93 (d, 1H, J = 6.0 Hz, 3-CH), 4.16 and 4.22 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.32 (m, 1H, 7-CH), 4.37 (br s, 1H, 10-OH), 4.58 (t, 1H, J = 5.0 Hz, 2′-CH), 4.95 (d, 1H, J = 5.2 Hz, 3′-CH), 4.95 (m, 1H, -CONH-), 4.97 (d, 1H, J = 7.6 Hz, 5-CH), 5.24 (d, 1H, J = 2.0 Hz,10-CH), 5.69 (d, 1H, J = 7.2 Hz, 2-CH), 6.20 (t, 1H, J = 9.0 Hz, 13-CH), 7.22 (m, 3H, 3′-Ph), 7.28 (d, 1H, J = 6.8 Hz, 3′-Ph), 7.53 (t, 2H, J = 7.6 Hz, m-OBz), 7.58 (d, 4H, J = 8.4 Hz, -PhBr), 7.67 (t, 1H, J = 7.2 Hz, p-OBz), 8.12 (d, 2H, J = 7.2 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.52, 171.81, 170.04, 165.74, 140.96, 137.43, 137.34, 136.76, 133.17, 131.68, 130.48, 130.00, 128.71, 128.49, 128.10, 127.71, 127.58, 126.11, 84.16, 80.86, 77.87, 75.97, 75.23, 74.86, 74.27, 71.52, 71.24, 60.67, 57.64, 46.51, 43.26, 36.68, 36.13, 26.28, 22.19, 20.63, 13.55, 9.53; HRMS (ESI) m/z calcd. for C44H48BrNO14SNa+ [M+Na+]: 948.1877, found 948.1870.
N-De-tert-butoxycarbonyl-N-(2,4,6-trimethyl)-phenylsulfonyl docetaxel (3j). White powder; Yield 65% (110 mg); mp 165–167 °C; 1H-NMR (CDCOCD3): δ 1.13 (s, 3H, 17-CH3), 1.21 (s, 3H, 16-CH3), 1.73 (s, 3H, 19-CH3), 1.87 (s, 3H, 18-CH3), 2.16 (m, 2H, 14-CH2), 2.20 (s, 3H, p-3″-CH3), 2.36 (s, 3H, OAc), 1.84 and 2.45 (2m, 2H, 6-CH2), 2.55 (s, 6H, o-3″-CH3), 3.83 (s, 1H, 2′-OH), 3.90 (d, 1H, J = 7.2 Hz, 3-CH), 4.16 and 4.19 (2d, 2H, J = 8.0 Hz, 20-CH2), 4.30 (m, 1H, 7-CH), 4.35 (br s, 1H, 10-OH), 4.53 (t, 1H, J = 4.0 Hz, 2′-CH), 4.75 (d, 2H, J = 4.8 Hz, -CONH-), 4.96 (d, 1H, J = 9.6 Hz, 5-CH), 5.00 (m, 1H, 3′-CH), 5.23 (d, 1H, J = 2.0 Hz,10-CH), 5.68 (d, 1H, J = 7.2 Hz, 2-CH), 6.09 (t, 1H, J = 8.4 Hz, 13-CH), 6.85 (s, 2H, 3″-Ph), 7.17-7.28 (m, 5H, 3′-Ph), 7.61 (t, 2H, J = 8.0 Hz, m-OBz), 7.71 (t, 1H, J = 7.4 Hz, p-OBz), 8.11 (d, 2H, J = 7.6 Hz, o-OBz); 13C-NMR (CD3COCD3) δ 210.52, 171.94, 169.86, 165.72, 141.79, 138.47, 137.86, 137.33, 136.77, 135.17, 133.22, 131.57, 130.46, 129.91, 128.52, 127.97, 127.61, 127.36, 84.13, 80.86, 77.77, 77.67, 75.93, 75.13, 74.77, 74.29, 71.52, 71.32, 60.19, 57.63, 46.50, 43.24, 36.67, 35.82, 26.21, 22.36, 22.21, 20.49, 19.91, 13.46, 9.47. HRMS (ESI) m/z calcd. for C47H55NO14SNa+ [M+Na+]: 912.3241, found 912.3264.