Effect of Salicylic Acid and Structurally Related Compounds in the Accumulation of Phytoalexins in Cotyledons of Common Bean (Phaseolus vulgaris L.) Cultivars
Abstract
:1. Introduction
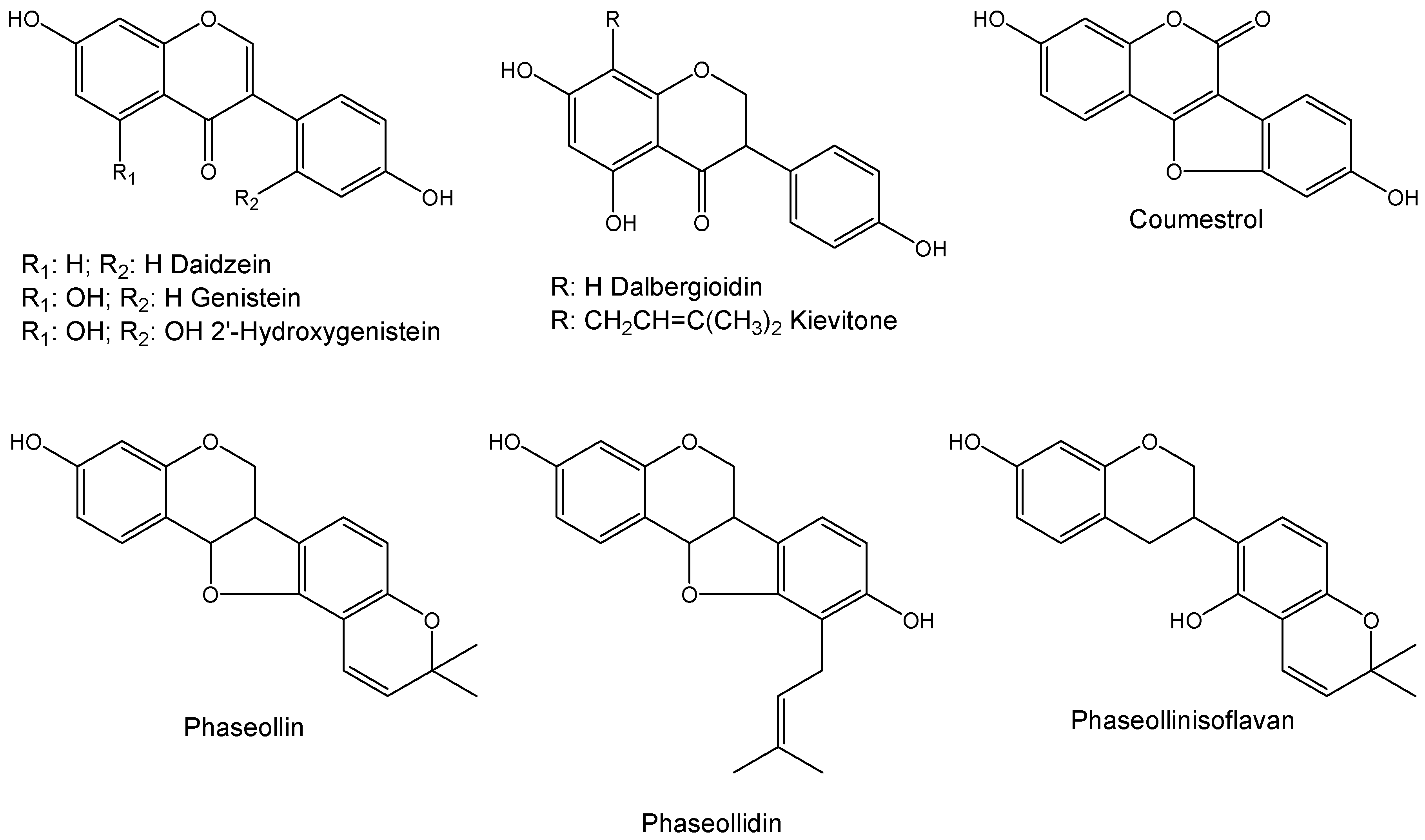
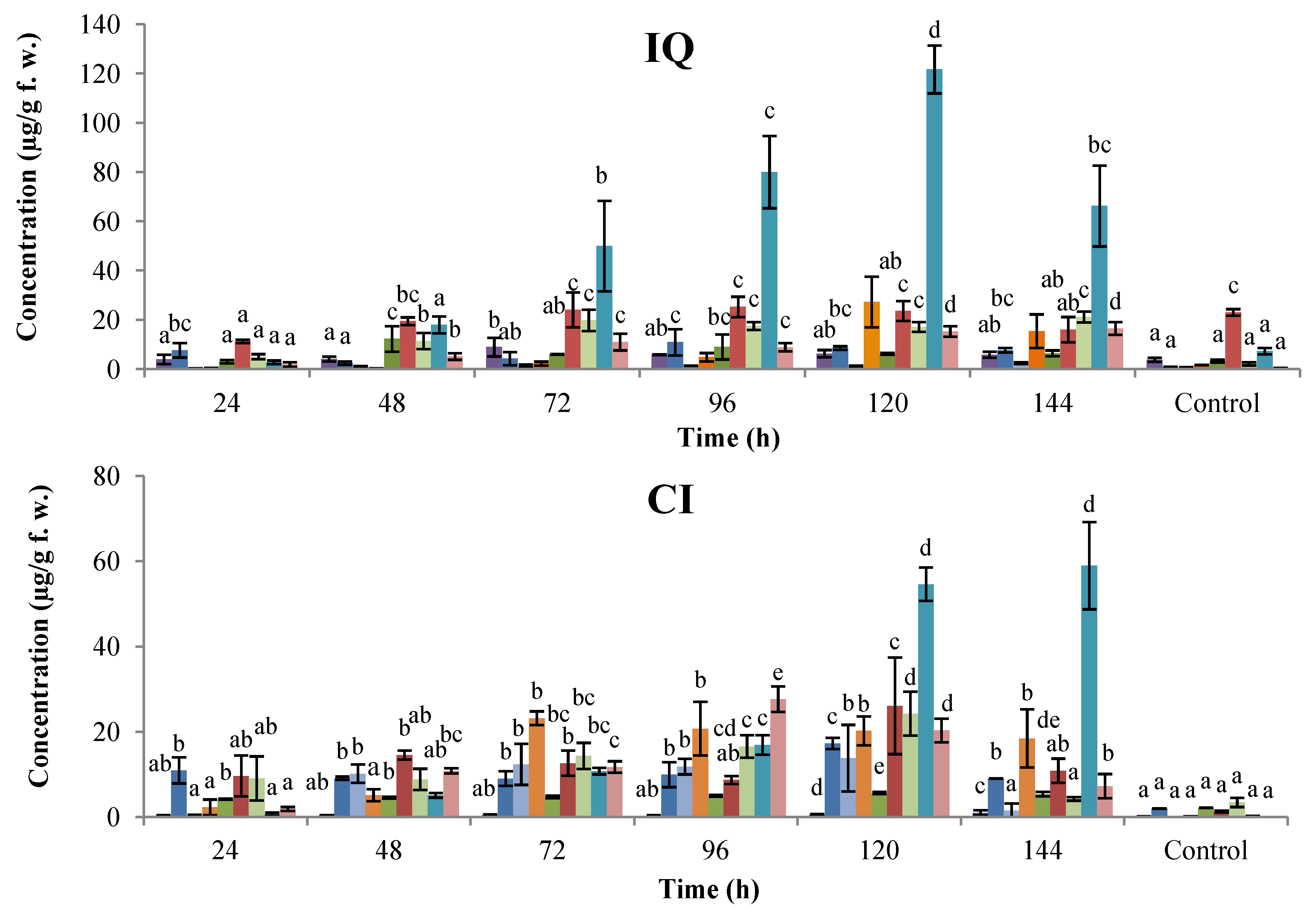
2. Results and Discussion
2.1. Time-Course Phytoalexin Accumulation
 , genistein;
, genistein;  , dalbergioidin;
, dalbergioidin;  , phaseollinisoflavan;
, phaseollinisoflavan;  , phaseollidin;
, phaseollidin;  , daidzein;
, daidzein;  , 2’-hydroxygenistein;
, 2’-hydroxygenistein;  , kievitone;
, kievitone;  , coumestrol;
, coumestrol;  , phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
, phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
 , genistein;
, genistein;  , dalbergioidin;
, dalbergioidin;  , phaseollinisoflavan;
, phaseollinisoflavan;  , phaseollidin;
, phaseollidin;  , daidzein;
, daidzein;  , 2’-hydroxygenistein;
, 2’-hydroxygenistein;  , kievitone;
, kievitone;  , coumestrol;
, coumestrol;  , phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
, phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).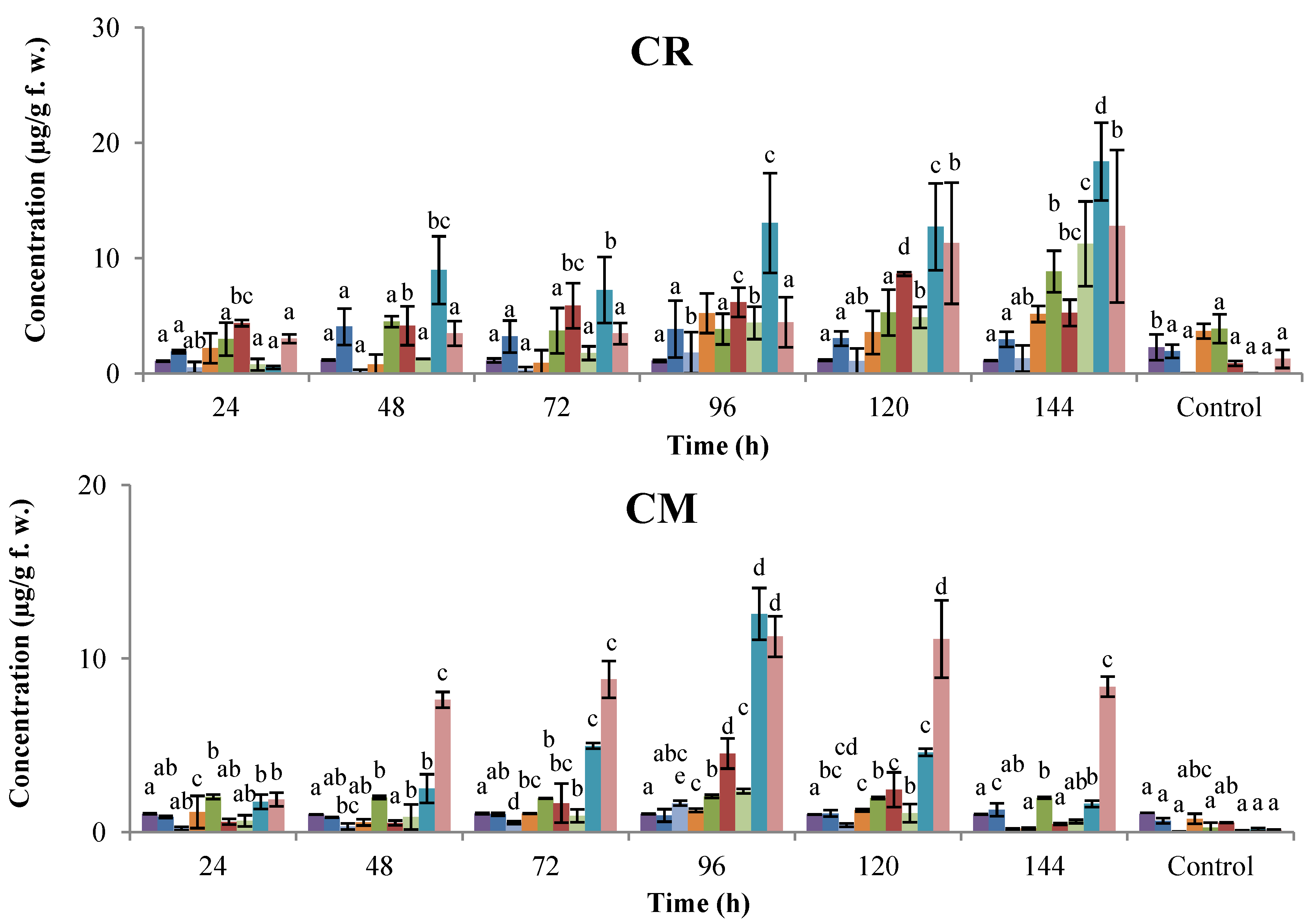
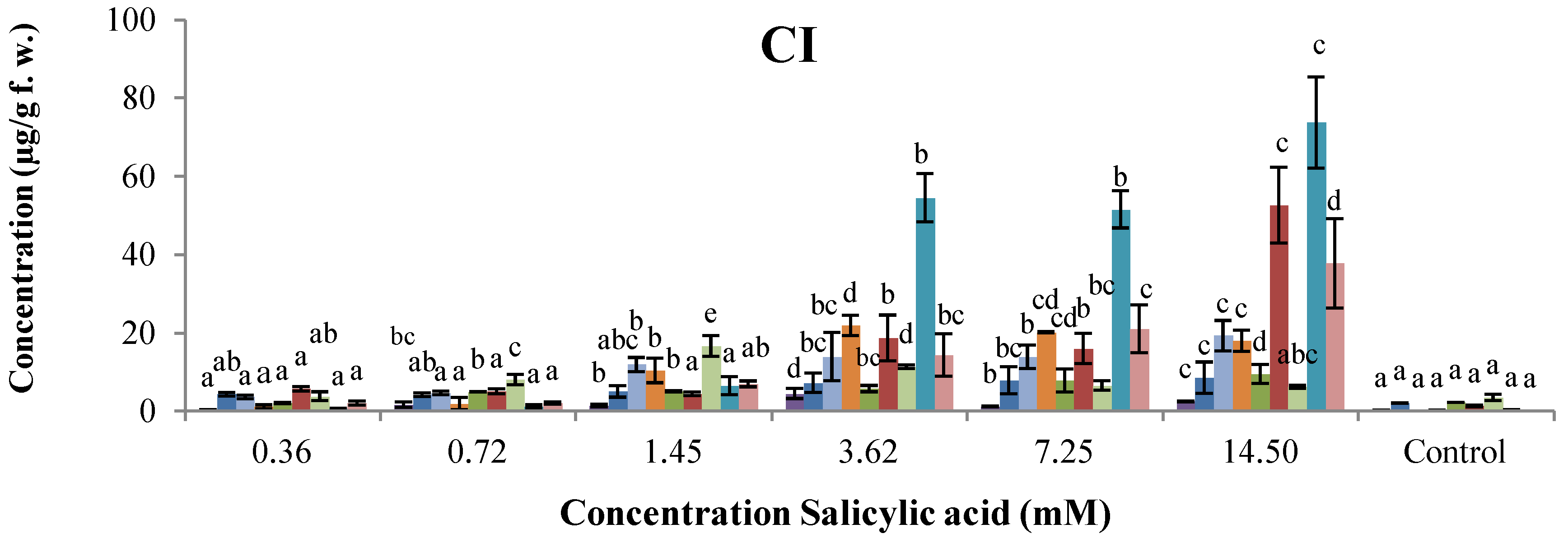
2.2. Dose–Response of Elicitor Treatments
 , genistein;
, genistein;  , dalbergioidin;
, dalbergioidin;  , phaseollinisoflavan;
, phaseollinisoflavan;  , phaseollidin;
, phaseollidin;  , daidzein;
, daidzein;  , 2’-hydroxygenistein;
, 2’-hydroxygenistein;  , kievitone;
, kievitone;  , coumestrol;
, coumestrol;  , phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
, phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
 , genistein;
, genistein;  , dalbergioidin;
, dalbergioidin;  , phaseollinisoflavan;
, phaseollinisoflavan;  , phaseollidin;
, phaseollidin;  , daidzein;
, daidzein;  , 2’-hydroxygenistein;
, 2’-hydroxygenistein;  , kievitone;
, kievitone;  , coumestrol;
, coumestrol;  , phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).
, phaseollin. Bars represent the mean concentrations of the isoflavonoids ± standard deviation (n = 3). Cultivars: CM, Cargamanto Mocho; CR, Cargamanto Rojo; IQ, ICA Quimbaya; CI, CORPOICA 106. For each compound, bars with different letters are significantly different (p = 0.05; Fisher’s LSD test).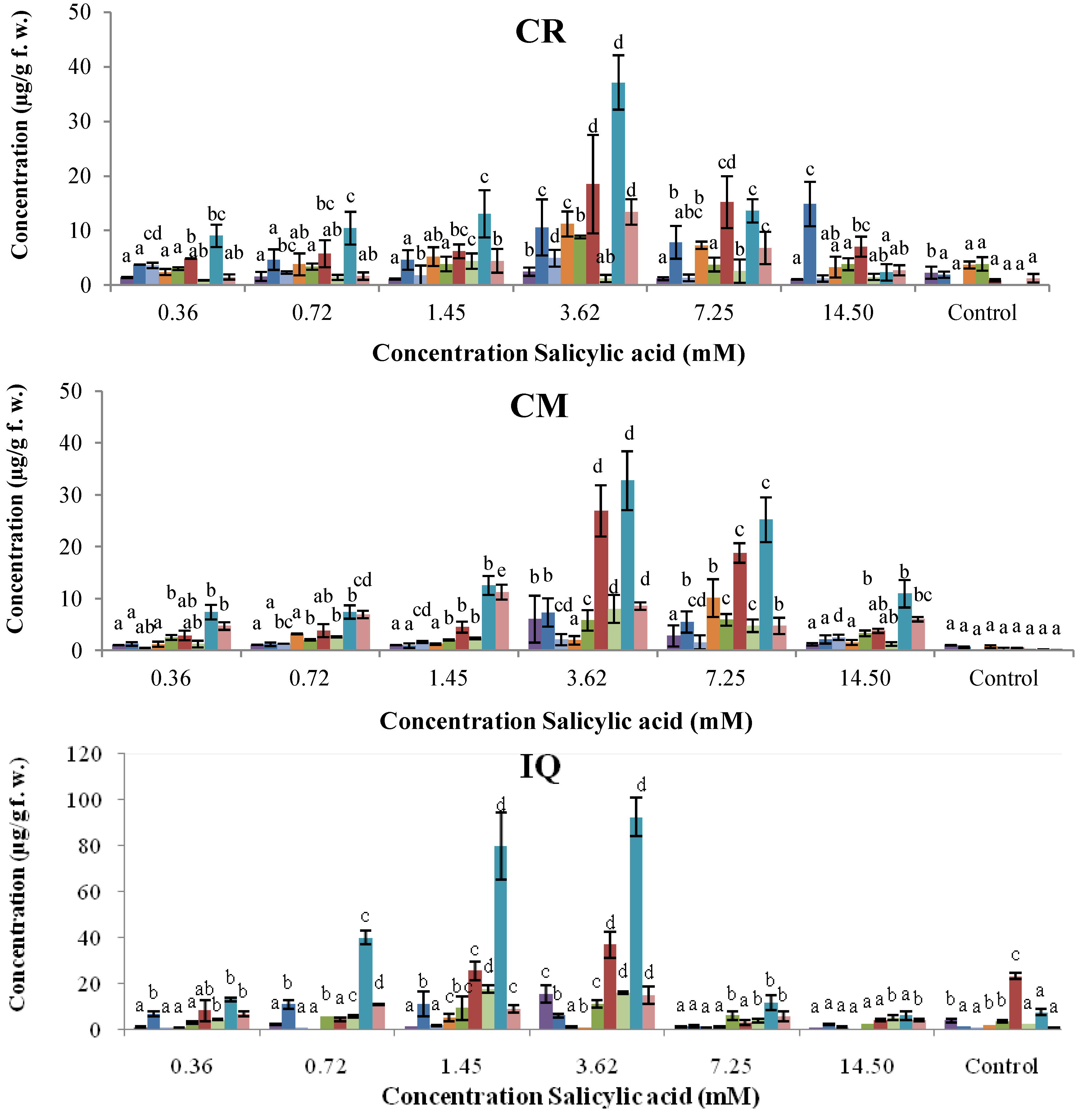
2.3. Antifungal Activity
 , solvent control;
, solvent control;  , CR-untreated cotyledons;
, CR-untreated cotyledons;  , CR-0.72 mM SA;
, CR-0.72 mM SA;  , CR-1.45 mM SA;
, CR-1.45 mM SA;  , CR-3.62 mM SA;
, CR-3.62 mM SA;  , IQ-untreated cotyledons;
, IQ-untreated cotyledons;  , IQ-0.72 mM SA;
, IQ-0.72 mM SA;  , IQ-1.45 mM SA;
, IQ-1.45 mM SA;  , IQ-3.62 mM SA. Figure 4b:
, IQ-3.62 mM SA. Figure 4b:  , solvent control;
, solvent control;  , CR-untreated cotyledons;
, CR-untreated cotyledons;  , CR-48 h post-induction;
, CR-48 h post-induction;  , CR-96 h post-induction;
, CR-96 h post-induction;  , CR-144 h post-induction;
, CR-144 h post-induction;  , IQ-untreated cotyledons;
, IQ-untreated cotyledons;  , IQ-48 h post-induction;
, IQ-48 h post-induction;  , IQ-96 h post-induction;
, IQ-96 h post-induction;  , IQ-144 h post-induction. For each time point, the bars headed by the same letter do not differ at p = 0.05 (Fisher’s LSD test).
, IQ-144 h post-induction. For each time point, the bars headed by the same letter do not differ at p = 0.05 (Fisher’s LSD test).
 , solvent control;
, solvent control;  , CR-untreated cotyledons;
, CR-untreated cotyledons;  , CR-0.72 mM SA;
, CR-0.72 mM SA;  , CR-1.45 mM SA;
, CR-1.45 mM SA;  , CR-3.62 mM SA;
, CR-3.62 mM SA;  , IQ-untreated cotyledons;
, IQ-untreated cotyledons;  , IQ-0.72 mM SA;
, IQ-0.72 mM SA;  , IQ-1.45 mM SA;
, IQ-1.45 mM SA;  , IQ-3.62 mM SA. Figure 4b:
, IQ-3.62 mM SA. Figure 4b:  , solvent control;
, solvent control;  , CR-untreated cotyledons;
, CR-untreated cotyledons;  , CR-48 h post-induction;
, CR-48 h post-induction;  , CR-96 h post-induction;
, CR-96 h post-induction;  , CR-144 h post-induction;
, CR-144 h post-induction;  , IQ-untreated cotyledons;
, IQ-untreated cotyledons;  , IQ-48 h post-induction;
, IQ-48 h post-induction;  , IQ-96 h post-induction;
, IQ-96 h post-induction;  , IQ-144 h post-induction. For each time point, the bars headed by the same letter do not differ at p = 0.05 (Fisher’s LSD test).
, IQ-144 h post-induction. For each time point, the bars headed by the same letter do not differ at p = 0.05 (Fisher’s LSD test).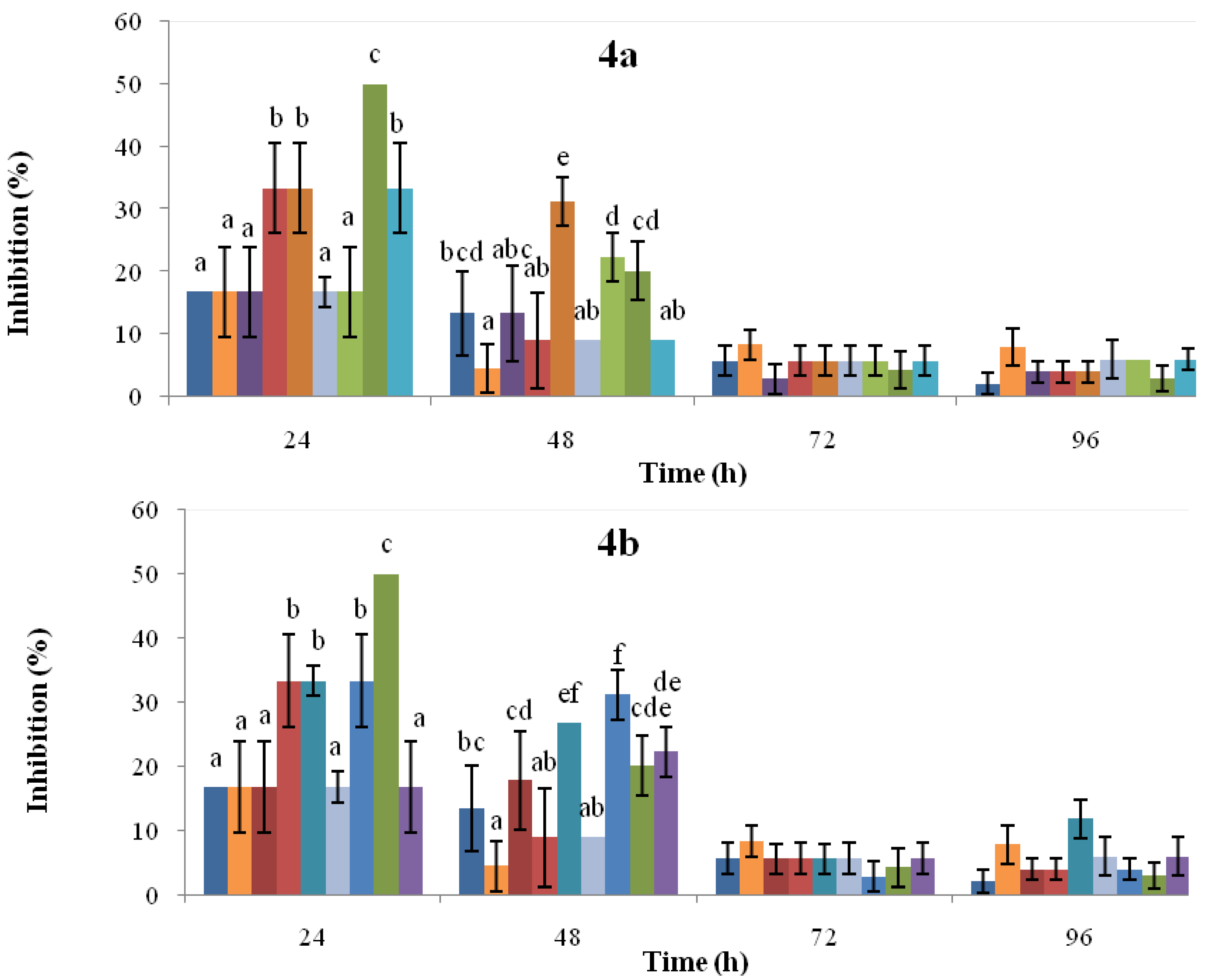
2.4. Inducer and Antifungal Effects of Structurally Related Compounds to SA
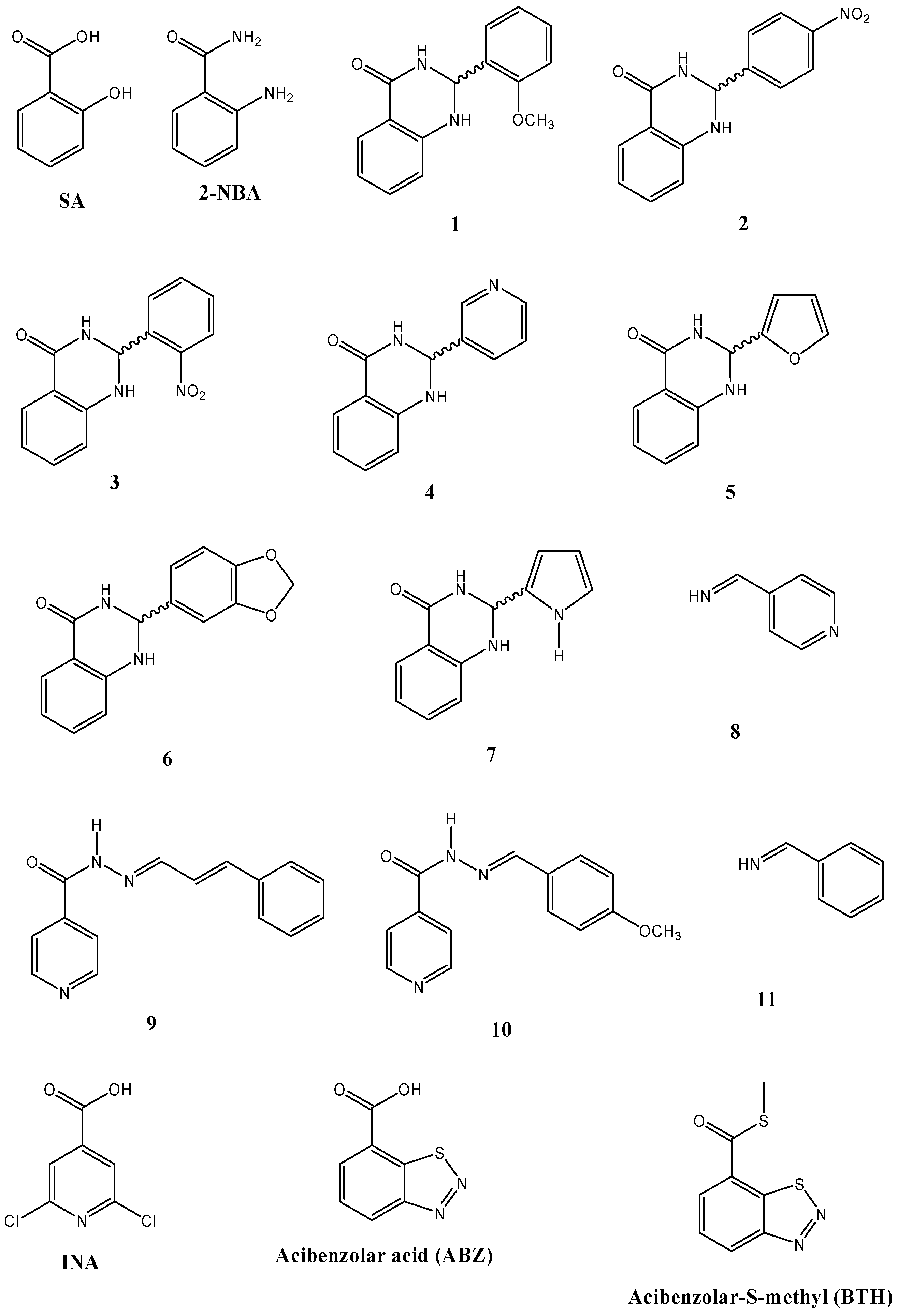
| Compound | Isoflavones/Isoflavanones * (µg/g f.w.) | Coumestan (µg/g f.w.) | Pterocarpans/Isoflavan † (µg/g f.w.) | Radial growth inhibition (%) of C. lindemuthianum | ||
|---|---|---|---|---|---|---|
| Elicited-cotyledon extracts | Elicitor | |||||
| 24 h | 48 h | 48 h | ||||
| SA | 20.20 ± 1.47 d | 13.07 ± 1.49 a | 11.53 ± 1.42 | n.d. | 9.5 ± 4.1 | |
| 2-NBA | 22.97 ± 2.33 a,c,d | 13.72 ± 1.12 a | 17.72 ± 5.45 | n.d. | n.d. | |
| 1 § | 43.04 ± 9.36 a,b,c,d | 79.99 ± 22.02 a,b,c,d | 46.67 ± 10.52 a,b,c,d | 82.4 ± 0.0 | 33.7 ± 19.8 | 38.5 ± 6.7 |
| 2 § | 15.57 ± 3.23 | 12.93 ± 6.68 a | 13.73 ± 2.85 | 38.2 ± 8.8 | 19.8 ± 3.5 | 11.5 ± 6.7 |
| 3 § | 20.16 ± 4.06 b,c | 14.37 ± 0.20 a | 19.92 ± 9.89 a,c | 38.2 ± 8.8 | 19.8 ± 3.5 | 42.3 ± 0.0 |
| 4 § | 24.13 ± 6.84 a,c,d | 14.98 ± 1.75 a | 34.90 ± 16.37 a,c,d | 47.1 ± 0.0 | 23.3 ± 0.0 | 53.8 ± 11.5 |
| 5 § | 20.81 ± 6.81 c | 21.25 ± 9.26 a | 11.94 ± 5.21 | 52.9 ± 5.1 | 26.7 ± 10.5 | 42.3 ± 0.0 |
| 6 § | 29.26 ± 14.19 a,b,c,d | 68.58 ± 18.89 a,b,c,d | 12.05 ± 8.51 | 55.9 ± 8.8 | 44.2 ± 20.9 | 57.7 ± 13.3 |
| 7 § | 17.64 ± 6.46 c | 24.81 ± 9.79 a | 36.03 ± 9.33 a,c,d | 64.7 ± 0.0 | 58.1 ± 7.0 | 38.5 ± 6.7 |
| 8 ‡ | 17.96 ± 2.86 a,b,c | 23.08 ± 3.11 a | 28.29 ± 5.00 a,c,d | 64.7 ± 0.0 | 33.7 ± 3.5 | 65.4 ± 0.0 |
| 9 ǂ | 32.60 ± 16.75 a,b,c,d | 18.02 ± 2.69 a | 26.96 ± 4.08 a,c,d | 47.1 ± 0.0 | 23.3 ± 0.0 | 50.0 ± 0.0 |
| 10 ǂ | 37.88 ± 11.98 a,b,c,d | 38.47 ± 4.40 a,b,c,d | 60.85 ± 17.05 a,b,c,d | 70.6 ± 10.2 | 45.3 ± 17.4 | 76.9 ± 0.0 |
| 11 ‡ | 13.85 ± 3.88 | 10.99 ± 3.01 a | 34.71 ± 6.11 a,c,d | 47.1 ± 17.6 | 33.7 ± 3.5 | 53.8 ± 0.0 |
| ABZ | 10.58 ± 1.58 | 17.74 ± 3.27 a,b | 9.32 ± 0.41 | n.d. | n.d. | |
| BHT | 7.90 ± 0.64 | 7.58 ± 1.83 a | 6.19 ± 1.57 b | n.d. | n.d. | |
| INA | 14.64 ± 2.53 | 18.58 ± 5.14 a | 22.61 ± 6.51 a | n.d. | n.d. | |
| Control | 9.01 ± 0.52 | Traces | 5.02 ± 0.33 b | n.d. | n.d. | |
3. Experimental
3.1. Reagents
3.2. Plant Material
3.3. Treatments
3.3.1. Dose-Response Experiments
3.3.2. Time-Course Experiments
3.3.3. Inducer Effect of Structurally Related Compounds to SA
3.4. Sample Preparation
3.5. HPLC Analysis
3.6. Identification and Quantification of Phytoalexins
3.7. Antifungal Assays
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- McConnell, M.; Mamidi, S.; Lee, R.; Chikara, S.; Rossi, M.; Papa, R.; McClean, P. Syntenic relationship among legumes revealed using a gene-based genetic linkage map of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2010, 121, 1103–1116. [Google Scholar] [CrossRef]
- Paredes, M.; Becerra, V.; Tay, J. Inorganic nutritional composition of common bean (Phaseolus vulgaris L.) genotypes race Chile. Chil. J. Agric. Res. 2009, 69, 486–495. [Google Scholar]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as alternative strategy to control post-harvest fungal rotting of fruits and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Induced disease resistance: How do induced plants stop pathogens? Physiol. Mol. Plant Pathol. 1999, 55, 77–84. [Google Scholar] [CrossRef]
- Edreva, A. A novel strategy for plant protection: Induced resistance. J. Cell Mol. Biol. 2004, 3, 61–69. [Google Scholar]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Heijari, J.; Nerg, A.M.; Vourinen, M.; Kainulainen, P. Potential for the use of exogenous chemical elicitors in disease and insect. Open For. Sc. J. 2009, 2, 17–24. [Google Scholar]
- Heil, M.; Bostock, R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defenses. Ann. Bot.-London 2002, 89, 503–512. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell. 1996, 8, 1809–1819. [Google Scholar]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Vimala, R.; Suriachandraselvan, M. Induced resistance in bhedi against powdery mildew by foliar application of salicylic acid. J. Biopest. 2009, 2, 111–114. [Google Scholar]
- Mandal, S. Induction of phenolics, lignin and key defense enzymes in eggplant (Solanum melongena L.) roots in response to elicitors. Afr. J. Biotechnol. 2010, 9, 8038–8047. [Google Scholar]
- Vernooij, B.; Friedrich, L.; Goy, P.A.; Staub, T.; Kessmann, H.; Ryals, J. 2,6-Dicloroisonicotinic acid-induced resistance to pathogens without the accumulation of salicylic acid. Mol. Plant Microbe Interact. 1995, 8, 228–234. [Google Scholar] [CrossRef]
- Kogel, K.H.; Backhove, U.; Romme, Y. Acquired resistance in barley: The resistance mechanisms induced by 2,6 dichloro-isonicotinic acid is a phenocopy of a genetically based mechanism governing race specific powdery mildew resistance. Plant Physiol. 1994, 106, 1267–1277. [Google Scholar]
- Metraux, J.P.; Ahl-Goy, P.; Staub, T.; Speich, J.; Steinemann, A.; Ryals, J.; Ward, E. Induced resistance in cucumber in response to 2, 6-dichloroisonicotinic acid and pathogens. In Advances in Molecular Genetics of Plant-Microbe Interactions, 1st ed.; Hennecke, H., Verma, D.P.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; Volume 1, pp. 432–439. [Google Scholar]
- Cole, D.L. The efficacy of acibenzolar-S-methyl, an inducer of systemic acquired resistance, against bacterial and fungal diseases of tobacco. Crop Prot. 1999, 18, 267–273. [Google Scholar] [CrossRef]
- Anfoka, G.H. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum Mill. cv. Volledung) to cucumber mosaic virus. Crop Prot. 2000, 19, 401–405. [Google Scholar] [CrossRef]
- Durango, D.; Quiñones, W.; Torres, F.; Rosero, Y.; Gil, J.; Echeverri, F. Phytoalexin accumulation in colombian bean varieties and aminosugars as elicitors. Molecules 2002, 7, 817–832. [Google Scholar] [CrossRef]
- Young, D.H.; Kohle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension-cultured Glycine max and Phaseolus vulgaris cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef]
- Longland, A.C.; Slusarenko, A.J.; Friend, J. Arachidonic and linoleic acids elicit isoflavonoid phytoalexin accumulation in Phaseolus vulgaris (French bean). J. Phytopathol. 1987, 120, 289–297. [Google Scholar] [CrossRef]
- Hynes, R.K.; Hill, J.; Reddy, M.S.; Lazarovits, G. Phytoalexin production by wounded white bean (Phaseolus vulgaris) cotyledons and hypocotyls in response to inoculation with rhizobacteria. Can. J. Microbiol. 1994, 40, 548–554. [Google Scholar] [CrossRef]
- Goossens, J.F.V.; Vendrig, J.C. Effects of abscisic acid, cytokinins, and light on isoflavonoid phytoalexin accumulation in Phaseolus vulgaris L. Planta 1982, 154, 441–446. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Dietrich, S.M.; Braga, M.R. Phytoalexin response of fifteen brazilian soybean cultivars. Rev. Bras. Fisiol. Veg. 2000, 12, 45–53. [Google Scholar]
- Kessmann, H.; Barz, W. Elicitation and suppression of phytoalexin and isoflavone accumulation in cotyledons of Cicer arietinum L., as caused by wounding and by polymeric components from the fungus Ascochyta rabiei. J. Phytopathol. 1986, 117, 321–335. [Google Scholar] [CrossRef]
- Christophe, S.; Zuanazzi, J.; El-Turk, J.; Leymarie, J.; Breda, C.; Buffard, D.; de Kozak, I.; Ratet, P.; Husson, P.; Kondorosi, A.; Esnault, R. Gene expression is not systemically linked to phytoalexin production during alfalfa leaf interaction with pathogenic bacteria. Mol. Plan-Microbe Interact. 1997, 10, 257–267. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Kollipara, S.S.; Vincent, J.R.; Lyons, P.C.; Cadena-Gomez, G. Phytoalexin synthesis by the sorghum mesocotyl in response to infection by pathogenic and nonpathogenic fungi. Proc. Natl. Acad. Sci. USA 1987, 84, 5520–5524. [Google Scholar] [CrossRef]
- Shettel, N.L.; Blake, N.E. Plant growth response to several allelopathic chemicals. Weed Sci. 1983, 31, 293–298. [Google Scholar]
- Pancheva, T.V.; Popova, L.P.; Uzunova, A.N. Effects of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef]
- Pedras, M.S.; Ahiahonu, P.W.K. Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry 2005, 66, 391–411. [Google Scholar] [CrossRef]
- Qian, Z.G.; Zhao, Z.J.; Xu, Y.; Qian, X.; Zhong, J.J. Novel synthetic 2,6-dichloroisonicotinate derivatives as effective elicitors for inducing the biosynthesis of plant secondary metabolites. Appl. Microbiol. Biot. 2006, 71, 164–167. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Qian, Z.; Tian, W.; Qian, X.; Zhong, J.J. Novel fluoro- and hydroxyl-containing jasmonate derivatives as highly efficient elicitors in suspension cultures of Taxus chinensis. Bioorg. Med. Chem. Lett. 2004, 14, 4755–4758. [Google Scholar] [CrossRef]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessman, H.; Ryals, J. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, Activates gene expression and disease resistance in barley. Plant Cell 1996, 8, 629–643. [Google Scholar]
- Dolezal, M.; Tumova, L.; Kesetovicová, D.; Tuma, J.; Králová, K. Substituted N-phenylpyrazine-2-carboxamides, their synthesis and evaluation as herbicides and abiotic elicitors. Molecules 2007, 12, 2589–2598. [Google Scholar] [CrossRef]
- Lenka, T.; Tuma, J.; Dolezal, M. Pyrazinecarboxamides as potential elicitors of flavonolignan and flavonoid production in Silybum marianum and Ononis arvensis cultures in vitro. Molecules 2011, 16, 9142–9152. [Google Scholar] [CrossRef]
- Kasparová, M.; Siatka, T.; Klimezová, V.; Dusek, J. New synthetic pyridine derivate as potential elicitor in production of isoflavonoids and flavonoids in Trifolium pratense L. suspension culture. Sci. World J. 2012, 74, 1–5. [Google Scholar]
- Knoth, C.; Salus, M.S.; Girke, T.; Eulgem, T. The synthetic elicitor 3,5-dichloroanthranilic acid induces NPR1-dependent and NPR1-independent mechanisms of disease resistance in Arabidopsis. Plant Physiol. 2009, 150, 333–347. [Google Scholar] [CrossRef]
- Jinichiro, K.; Toyozo, Y.; Umenura, K.; Iwata, M. Methods of screening elicitor inducing the production of phytoalexin in rice and rice disease controlling agent containing elicitor as the active ingredient. U.S. Patent 6,146,893; filed 21 April 1997, and issued 14 November 2000,
- Sahu, R.; Thakur, D.J.; Kashyap, P. Schiff base: An overview of its medicinal chemistry potential for new drug molecules. Int. J. Pharm. Sci. Nanotech. 2012, 5, 1757–1764. [Google Scholar]
- Rakhi, R.; Prasson, M.A. A review on biological activity of quinazolinones. Int. J. Pharm. Pharm. Sci. 2012, 4, 66–70. [Google Scholar]
- Pineda, R.; Vizcaíno, S.; García, C.M.; Gil, J.H.; Durango, D.L. Chemical composition and antifungal activity of Piper auritum Kunth and Piper holtonii C. DC. against phytopathogenic fungi. Chil. J. Agric. Res. 2012, 72, 507–515. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 to 11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Durango, D.; Pulgarin, N.; Echeverri, F.; Escobar, G.; Quiñones, W. Effect of Salicylic Acid and Structurally Related Compounds in the Accumulation of Phytoalexins in Cotyledons of Common Bean (Phaseolus vulgaris L.) Cultivars. Molecules 2013, 18, 10609-10628. https://doi.org/10.3390/molecules180910609
Durango D, Pulgarin N, Echeverri F, Escobar G, Quiñones W. Effect of Salicylic Acid and Structurally Related Compounds in the Accumulation of Phytoalexins in Cotyledons of Common Bean (Phaseolus vulgaris L.) Cultivars. Molecules. 2013; 18(9):10609-10628. https://doi.org/10.3390/molecules180910609
Chicago/Turabian StyleDurango, Diego, Natalia Pulgarin, Fernando Echeverri, Gustavo Escobar, and Winston Quiñones. 2013. "Effect of Salicylic Acid and Structurally Related Compounds in the Accumulation of Phytoalexins in Cotyledons of Common Bean (Phaseolus vulgaris L.) Cultivars" Molecules 18, no. 9: 10609-10628. https://doi.org/10.3390/molecules180910609




