Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus aurantium L. Growing in Greece
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition
| Nr | Compounds a | RT b | Retention Index c | Concentration % d,e | |||
|---|---|---|---|---|---|---|---|
| Flowers | Peel | Young Leaves | Old Leaves | ||||
| 1 | α-Pinene | 7.722 | 934 (939) | 1.35 ± 0.01 | 0.53 ± 0.02 | 0.19 ± 0.00 | - |
| 2 | Sabinene | 9.542 | 973 (976) | 2.01 ± 0.20 | 0.18 ± 0.01 | 0.37 ± 0.00 | 0.22 ± 0.03 |
| 3 | β-Pinene | 9.750 | 980 (980) | 19.08 ± 0.18 | 0.62 ± 0.04 | 3.58 ± 0.01 | 1.90 ± 0.31 |
| 4 | Myrcene | 10.509 | 991 (991) | 1.59 ± 0.01 | 2.00 ± 0.04 | 1.63 ± 0.06 | 2.74 ± 0.32 |
| 5 | Octanal | 10.953 | 999 (1001) | - | 0.24 ± 0.01 | - | - |
| 6 | 3-δ-Carene | 11.816 | 1014 (1011) | 0.17 ± 0.00 | - | - | - |
| 7 | Limonene | 12.630 | 1029 (1031) | 12.04 ± 0.16 | 94.67 ± 0.01 | 0.53 ± 0.01 | 0.77 ± 0.09 |
| 8 | cis-β-Ocimene | 13.206 | 1039 (1040) | 0.77 ± 0.02 | 0.30 ± 0.01 | 0.71 ± 0.02 | 1.22 ± 0.07 |
| 9 | trans-β-Ocimene | 13.884 | 1049 (1050) | 6.06 ± 0.01 | - | 4.08 ± 0.05 | 3.11 ± 0.21 |
| 10 | γ-Terpinene | 14.550 | 1060 (1062) | 0.36 ± 0.01 | - | - | - |
| 11 | Linalool oxide | 15.353 | 1071 (1074) | 0.29 ± 0.02 | - | - | - |
| 12 | α-Terpinolene | 16.440 | 1086 (1088) | 0.47 ± 0.01 | - | 0.40 ± 0.01 | 0.70 ± 0.04 |
| 13 | Linalool | 17.729 | 1102 (1104) | 29.14 ± 0.38 | 0.76 ± 0.04 | 58.21 ± 0.37 | 36.03 ± 0.60 |
| 14 | Terpin 4-ol | 23.122 | 1174 (1177) | 0.68 ± 0.02 | - | 0.17 ± 0.01 | 0.13 ± 0.02 |
| 15 | α-Terpineol | 24.267 | 1187 (1189) | 4.56 ± 0.05 | 0.13 ± 0.01 | 7.11 ± 0.06 | 12.89 ± 0.37 |
| 16 | Decanal | 25.574 | 1202 (1204) | - | 0.16 ± 0.03 | - | - |
| 17 | Nerol | 27.489 | 1227 (1228) | 0.83 ± 0.02 | - | 1.45 ± 0.02 | 2.89 ± 0.06 |
| 18 | Geraniol | 29.864 | 1257 (1255) | 4.31 ± 1.43 | - | - | - |
| 19 | Linalyl acetate | 30.963 | 1259 (1257) | 3.88 ± 0.40 | 0.18 ± 0.03 | 12.42 ± 0.13 | 23.00 ± 1.42 |
| 20 | Methyl anthranylate | 36.245 | 1334 (1337) | 0.19 ± 0.00 | - | - | - |
| 21 | δ-Elemene | 36.458 | 1337 (1339) | 0.12 ± 0.00 | - | - | - |
| 22 | Terpinyl acetate | 37.426 | 1349 (1350) | 0.20 ± 0.00 | - | - | 0.11 ± 0.00 |
| 23 | Neryl acetate | 38.901 | 1368 (1365) | 1.30 ± 0.01 | 0.10 ± 0.00 | 2.18 ± 0.07 | 4.46 ± 0.22 |
| 24 | Geranyl acetate | 40.419 | 1386 (1383) | 2.59 ± 0.04 | - | 4.49 ± 0.11 | 8.70 ± 0.37 |
| 25 | β-Caryophyllene | 42.266 | 1412 (1418) | 0.42 ± 0.01 | - | 1.09 ± 0.02 | 0.22 ± 0.01 |
| 26 | α-Humulene | 44.268 | 1447 (1454) | - | - | 0.10 ± 0.00 | - |
| 27 | (E)-β-Farnesene | 44913 | 1458 (1458) | - | - | 0.13 ± 0.01 | - |
| 28 | δ-Germacrene | 45.948 | 1476 (1480) | 0.13 ±0.01 | - | - | - |
| 29 | Bicyclogermacrene | 46.764 | 1489 (1494) | - | - | 0.18 ± 0.00 | 0.20 ± 0.01 |
| 30 | (E)-Nerolidol | 50.451 | 1566 (1564) | 1.76 ± 0.03 | - | - | 0.10 ± 0.00 |
| 31 | (E)-Farnesol | 55.544 | 1725 (1722) | 5.14 ± 0.02 | - | - | - |
| Total percentage d | 99.44% | 99.87% | 99.02% | 99.39% | |||
| Essential oil (%) content | 0.12 ± 0.01 | 1.67 ± 0.07 | 0.27 ± 0.01 | 0.45 ± 0.02 | |||
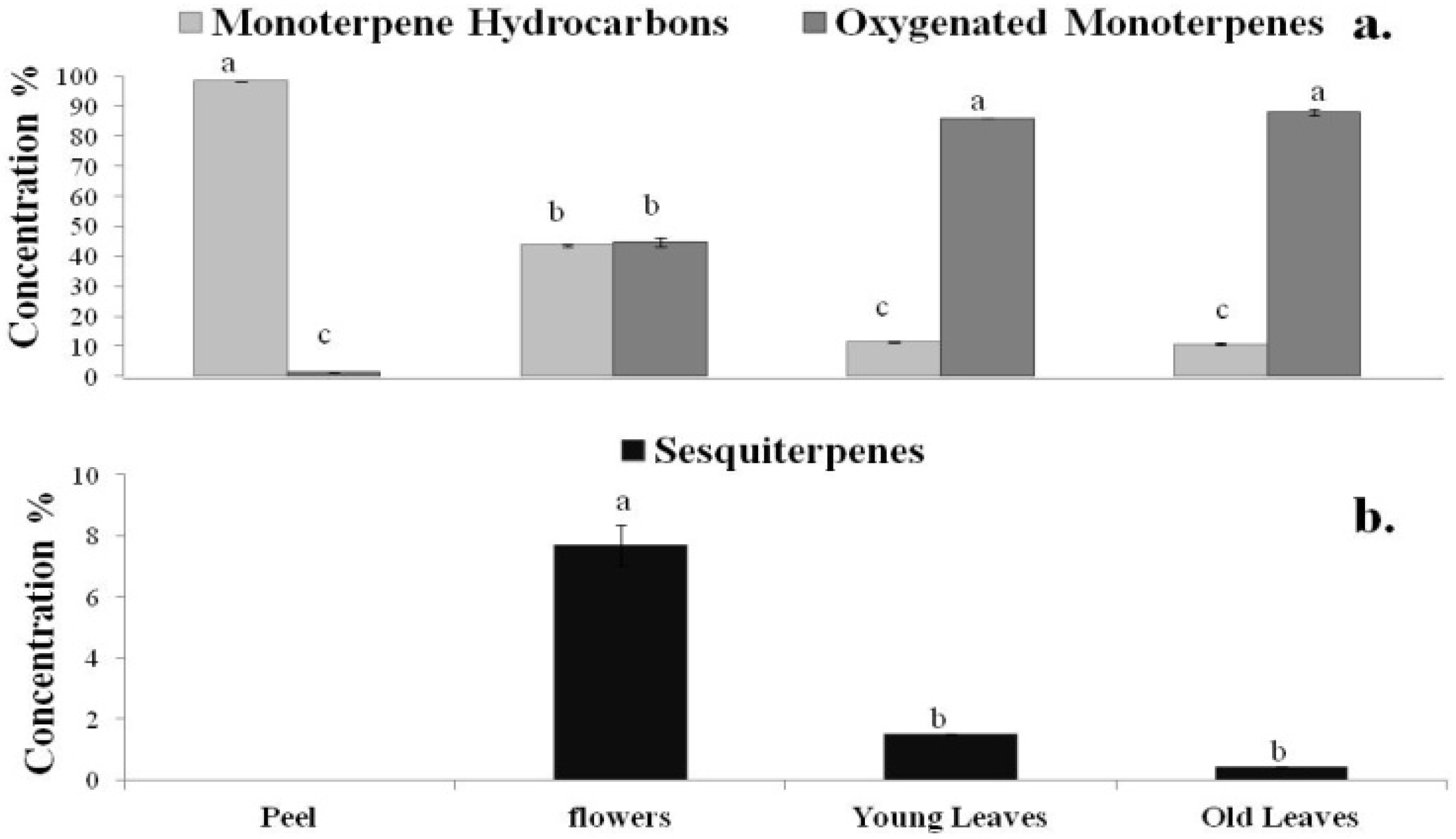
2.2. DPPH Radical Scavenging Activity
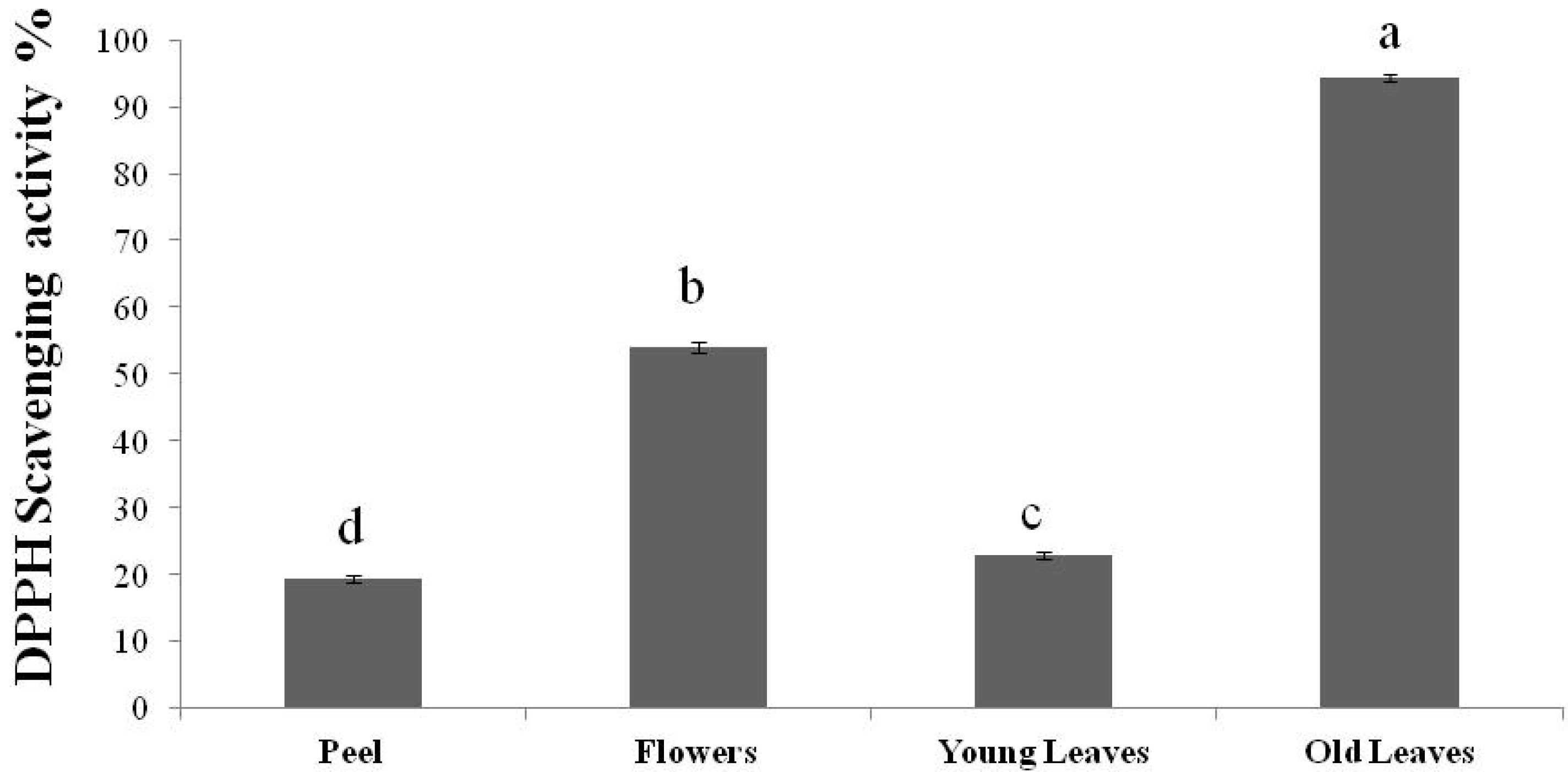
3. Experimental
3.1. Chemicals
3.2. Plant Material
3.3. Determination of Essential Oils
3.4. GC-MS Analysis
3.5. Identification of Components
3.6. DPPH Radical Scavenging Activity Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Al-Ababneh, S.S.; Karam, N.S.; Shibli, R.A. Cryopreservation of sour orange (Citrus aurantium L.) shoot tips. In Vitro Cell. Dev. Biol. Plant 2002, 38, 602–607. [Google Scholar] [CrossRef]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Machado Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from (Citrus aurantium) on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Kiple, K.F.; Ornelas, K.C. The Cambridge World History of Food; Cambridge University Press: Cambridge, UK, 2000; pp. 1822–1826. [Google Scholar]
- Fugh-Berman, A.; Myers, A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: Current status of clinical and basic research. Exp. Biol. Med. 2004, 229, 698–704. [Google Scholar]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Ramadan, W.; Mourad, B.; Ibrahim, S.; Sonbol, F. Oil of bitter orange: New topical antifungal agent. Int. J. Dermatol. 1996, 35, 448–449. [Google Scholar]
- Sonbol, F.; Ibrahim, S.M.; Mohamed, B.M. Antimicrobial activity of oil of bitter orange. Alex. J. Pharm. Sci. 1992, 9, 107–109. [Google Scholar]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1, 1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Azadi, B.; Nickavar, B.; Amin, G. Volatile constituents of the peel and leaf of Citrus aurantium L. cultivated in the north of Iran. J. Pharm. Health Sci. 2012, 1, 37–41. [Google Scholar]
- Njoroge, S.M.; Ukeda, H.; Kusunose, H.; Sawamura, M. Volatile components of the essential oils from kabosu, daidai, and yuko, Japanese sour Citrus fruits. Flavour Fragr. J. 1994, 9, 289–297. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Cogliandro, E.; Verzera, A.; Dugo, G. On the genuineness of citrus essential oils. 51. Oxygen heterocyclic compounds of bitter orange oil (Citrus aurantium L.). J. Agric. Food Chem. 1996, 44, 544–549. [Google Scholar] [CrossRef]
- Lota, M.L.; de Rocca Serra, D.; Jacquemond, C.; Tomi, F.; Casanova, J. Chemical variability of peel and leaf essential oils of sour orange. Flavour Fragr. J. 2001, 16, 89–96. [Google Scholar] [CrossRef]
- Kirbaşlar, F.G.; Tavman, A.; Dülger, B.; Türker, G. Antimicrobial activity of Turkish Citrus peel oils. Pak. J. Bot. 2009, 41, 3207–3212. [Google Scholar]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Ben Brahim, N.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Amanzade, Y.; Alhani, Z.; Hajimehdipour, H.; Faramarzi, M.A. GC/MS analysis of Citrus aurantium L. Hydrolate and its comparison with the commercial samples. Iran. J. Pharm. Res. 2004, 3, 177–179. [Google Scholar]
- Boussaada, O.; Chemli, R. Chemical composition of essential oils from flowers, leaves and peel of Citrus aurantium L. var. amara from Tunisia. J. Essent. Oil Bear. Plants 2006, 9, 133–139. [Google Scholar] [CrossRef]
- Hérent, M.F.; De Bie, V.; Tilquin, B. Determination of new retention indices for quick identification of essential oils compounds. J. Pharm. Biomed. Anal. 2007, 43, 886–892. [Google Scholar] [CrossRef]
- Seok, B.J.; Kim, S.S.; Lee, J.A.; Oh, T.H.; Kim, J.Y.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of essential oils extracted from Korean Endemic Citrus Species. J. Microbiol. Biotechnol. 2008, 18, 74–79. [Google Scholar]
- Malhotra, S.; Suri, S.; Tuli, R. Antioxidant activity of citrus cultivars and chemical composition of Citrus karna essential oil. Planta Med. 2009, 75, 62–64. [Google Scholar] [CrossRef]
- Hamdan, D.; El-Readi, M.Z.; Nibret, E.; Sporer, F.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition of the essential oils of two Citrus species and their biological activities. Pharmazie 2010, 65, 141–147. [Google Scholar]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Kumar Mishra, P.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef]
- Song, H.S.; Ukeda, H.; Sawamura, M. Antioxidative activities of Citrus peel essential oils and their components against linoleic acid oxidation. Food Sci. Technol. Res. 2001, 7, 50–56. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. Peel essential oils. J. Food Sci. 2012, 77, 40–46. [Google Scholar]
- De Pasquale, F.; Siragusa, M.; Abbate, L.; Tusa, N.; De Pasquale, C.; Alonzo, G. Characterization of five sour orange clones through molecular markers and leaf essential oils analysis. Sci. Hortic. 2006, 109, 54–59. [Google Scholar] [CrossRef]
- Kamal, G.M.; Anwar, F.; Hussain, A.I.; Sarri, N.; Ashraf, M.Y. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011, 18, 1275–1282. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Co.: Carol Stream, IL, USA, 1995. [Google Scholar]
- Su, M.; Silva, J.L. Antioxidant activity, anthocyanins and phenolics of rabbiteye blueberry (Vaccinium ashei) byproducts as affected by fermentation. Food Chem. 2006, 97, 447–451. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the essential oils are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus aurantium L. Growing in Greece. Molecules 2013, 18, 10639-10647. https://doi.org/10.3390/molecules180910639
Sarrou E, Chatzopoulou P, Dimassi-Theriou K, Therios I. Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus aurantium L. Growing in Greece. Molecules. 2013; 18(9):10639-10647. https://doi.org/10.3390/molecules180910639
Chicago/Turabian StyleSarrou, Eirini, Paschalina Chatzopoulou, Kortessa Dimassi-Theriou, and Ioannis Therios. 2013. "Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus aurantium L. Growing in Greece" Molecules 18, no. 9: 10639-10647. https://doi.org/10.3390/molecules180910639




