Antimycobacterial and Photosynthetic Electron Transport Inhibiting Activity of Ring-Substituted 4-Arylamino-7-Chloroquinolinium Chlorides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
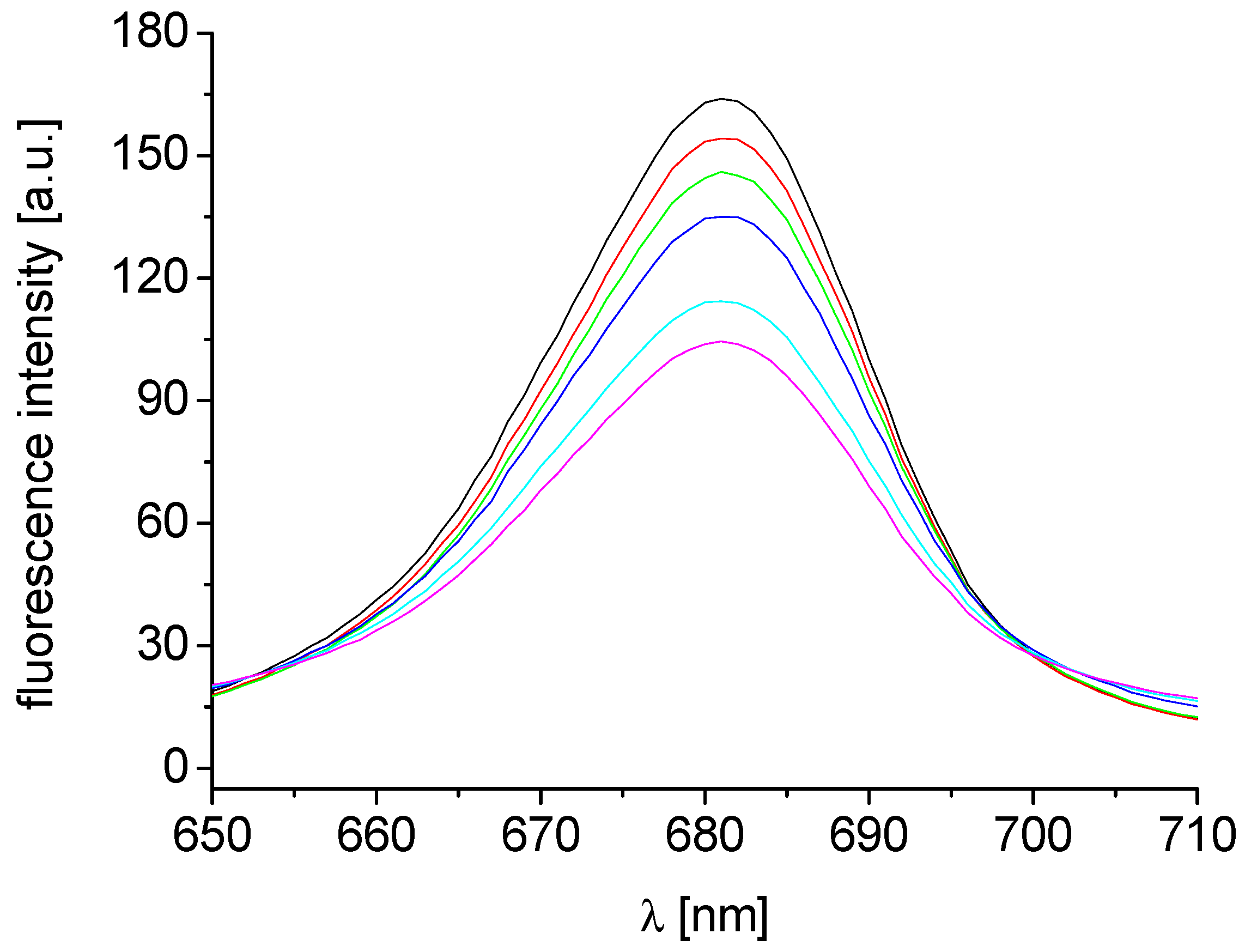
2.2. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
| Comp. | R | log P a | σ a | [μmol/L] | |||||
|---|---|---|---|---|---|---|---|---|---|
| PET IC50 | MIC | LD50 | |||||||
| MM | MK | MS | MAP | ||||||
| 1 | H | 4.19 | 0 | 469 | >879 | >219 | >879 | >859 | – |
| 2a | 2-OH | 3.61 | −0.38 | ND | 834 | 208 | 104 | 813 | – |
| 2b | 3-OH | 3.89 | 0.12 | ND | >834 | >834 | >834 | >813 | – |
| 2c | 4-OH | 3.71 | −0.37 | ND | >834 | >834 | >834 | >813 | – |
| 3a | 2-OCH3 | 4.66 | −0.28 | ND | >797 | >797 | 99.3 | 389 | – |
| 3b | 3-OCH3 | 4.66 | 0.12 | 238 | >797 | >797 | >797 | >778 | – |
| 3c | 4-OCH3 | 4.66 | −0.27 | ND | >797 | >797 | >797 | >778 | – |
| 4a | 2-CH3 | 4.45 | −0.17 | 411 | >839 | >839 | 52.4 | 159 | >20 |
| 4b | 3-CH3 | 4.76 | −0.07 | ND | ND | ND | ND | ND | – |
| 4c | 4-CH3 | 4.39 | −0.17 | ND | ND | ND | ND | ND | – |
| 5a | 2-F | 4.58 | 0.06 | 478 | >828 | 828 | 103 | >809 | – |
| 5b | 3-F | 4.29 | 0.34 | 116 | ND | ND | ND | ND | – |
| 5c | 4-F | 4.47 | 0.06 | 370 | ND | ND | ND | ND | – |
| 6a | 2-Cl | 4.41 | 0.22 | 362 | 98.6 | 49.1 | 196 | 383 | >20 |
| 6b | 3-Cl | 4.50 | 0.37 | 55 | ND | ND | ND | ND | – |
| 6c | 4-Cl | 4.18 | 0.23 | 211 | ND | ND | ND | ND | – |
| 7a | 2-Br | 4.89 | 0.22 | 251 | 86.5 | 43.2 | 86.5 | >675 | >20 |
| 7b | 3-Br | 5.14 | 0.39 | 89 | ND | ND | ND | ND | – |
| 7c | 4-Br | 4.89 | 0.23 | 128 | ND | ND | ND | ND | – |
| 8a | 2-CF3 | 4.81 | 0.51 | 367 | 177 | 177 | 178 | 330 | – |
| 8b | 3-CF3 | 5.23 | 0.43 | 27 | ND | ND | ND | ND | >20 |
| 8c | 4-CF3 | 5.05 | 0.51 | 33 | ND | ND | ND | ND | >20 |
| 9a | 2-NO2 | 5.10 | 0.77 | 132 | 380 | 380 | 380 | 743 | – |
| 9b | 3-NO2 | 5.16 | 0.71 | ND | ND | ND | ND | ND | – |
| 9c | 4-NO2 | 5.01 | 0.78 | ND | ND | ND | ND | ND | – |
| DCMU | – | – | – | 1.9 | – | – | – | – | – |
| INH | – | – | – | – | 467 | 29.2 | 117 | >1823 | – |
| PZA | – | – | – | – | – | – | – | >2031 | – |
| RIF | – | – | – | – | – | – | – | >109 | – |
| CPX | – | – | – | – | – | – | – | 181 | – |
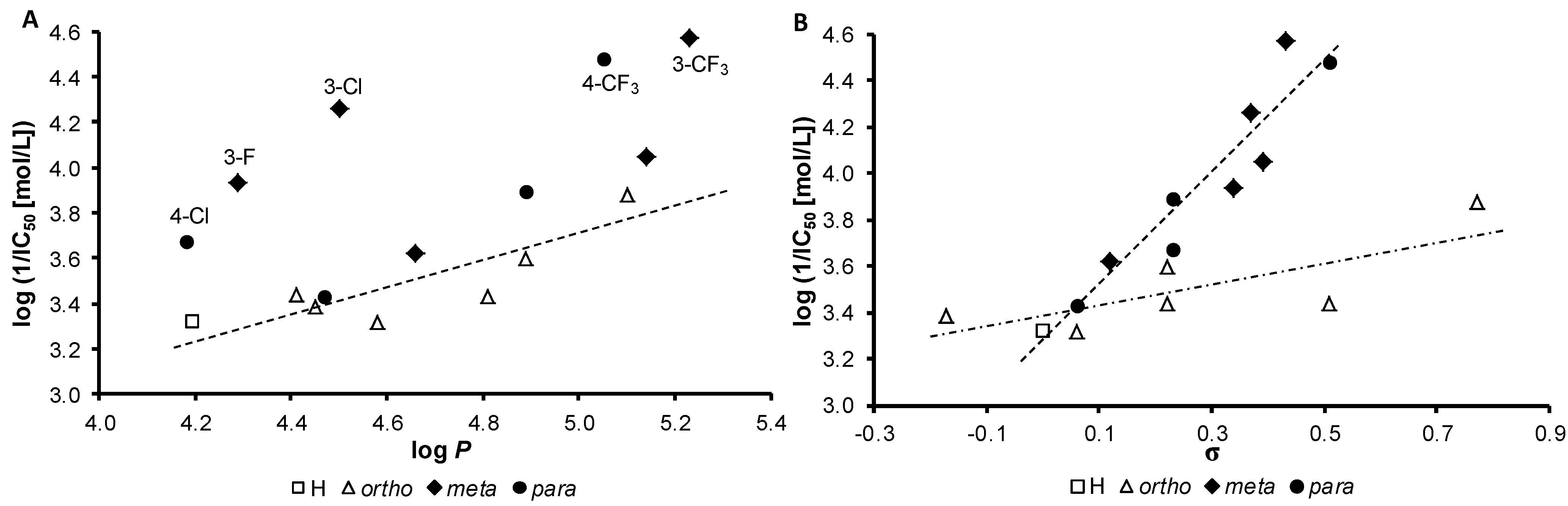
r = 0.797, s = 0.131, F = 8.7, n = 7
r = 0.945, s = 0.089, F = 67.0, n = 10
2.3. In Vitro Antimycobacterial Evaluation

2.4. In vitro Cytotoxicity Assay
3. Experimental
3.1. General
3.2. Synthesis
3.3. QSAR Study
3.4. Study of Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3.5. Study of Chlorophyll a Fluorescence in Spinach Chloroplasts
3.6. In Vitro Antimycobacterial Evaluation
3.7. In Vitro Cytotoxicity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2012; WHO Press: Geneva, Switzerland, 2012.
- Wagner, D.; Young, L.S. Nontuberculous mycobacterial infections: A clinical review. Infection 2004, 32, 257–270. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance 2001; WHO Press: Geneva, Switzerland, 2001.
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Acharya, N.; Varshney, U. Biochemical properties of single-stranded DNA-binding protein from Mycobacterium smegmatis, A fast-growing Mycobacterium and its physical and functional interaction with uracil DNA glycosylases. J. Mol. Biol. 2002, 318, 1251–1264. [Google Scholar] [CrossRef]
- Broussard, G.W.; Ennis, D.G. Mycobacterium marinum produces long-term chronic infections in medaka: A new animal model for studying human tuberculosis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 45–54. [Google Scholar] [CrossRef]
- Valente, W.J.; Pienaar, E.; Fast, A.; Fluitt, A.; Whitney, S.E.; Fenton, R.J.; Barletta, R.G.; Chacon, O.; Viljoen, H.J. A kinetic study of in vitro lysis of Mycobacterium smegmatis. Chem. Eng. Sci. 2009, 64, 1944–1952. [Google Scholar] [CrossRef]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Resp. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef]
- Roth, H.J.; Fenner, H. Arzneistoffe, 3rd ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2000. [Google Scholar]
- Janin, Y.L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef]
- Rivers, E.C.; Mancera, R.L. New anti-tuberculosis drugs with novel mechanisms of action. Curr. Med. Chem. 2008, 15, 1956–1967. [Google Scholar] [CrossRef]
- Vangapandu, S.; Jain, M.; Jain, R.; Kaurb, S.; Singh, P.P. Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorg. Med. Chem. 2004, 12, 2501–2508. [Google Scholar] [CrossRef]
- Nayyar, A.; Monga, V.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolines. Bioorg. Med. Chem. 2007, 15, 626–640. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Buchta, V.; Silva, L.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Nycz, J.E.; Pesko, M.; Carroll, J.; Kralova, K.; Vejsova, M.; O’Mahony, J.; Coffey, A.; Mrozek, A.; Polanski, J. Investigating the activity spectrum for ring-substituted 8-hydroxyquinolines. Molecules 2010, 15, 288–304. [Google Scholar] [CrossRef]
- Gonec, T.; Bobal, P.; Sujan, J.; Pesko, M.; Guo, J.; Kralova, K.; Pavlacka, L.; Vesely, L.; Kreckova, E.; Kos, J.; et al. Investigating the spectrum of biological activity of substituted quinoline-2-carboxamides and their isosteres. Molecules 2012, 17, 613–644. [Google Scholar] [CrossRef]
- Serda, M.; Mrozek-Wilczkiewicz, A.; Jampilek, J.; Pesko, M.; Kralova, K.; Vejsova, M.; Musiol, R.; Polanski, J. Investigation of biological properties for (hetero)aromatic thiosemicarbazones. Molecules 2012, 17, 13483–13502. [Google Scholar] [CrossRef]
- Cieslik, W.; Musiol, R.; Nycz, J.; Jampilek, J.; Vejsova, M.; Wolff, M.; Machura, B.; Polanski, J. Contribution to investigation of antimicrobial activity of styrylquinolines. Bioorg. Med. Chem. 2012, 20, 6960–6968. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Koul, A.; Choidas, A.; Treder, M.; Tyagi, A.K.; Drlica, K.; Singh, Y.; Ullrich, A. Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J. Bacteriol. 2000, 182, 5425–5432. [Google Scholar]
- Koul, A.; Herget, T.; Kleb, B.; Ullrich, A. Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2004, 2, 189–202. [Google Scholar] [CrossRef]
- Manger, M.; Scheck, M.; Prinz, H.; von Kries, J.P.; Langer, T.; Saxena, K.; Schwalbe, H.; Furstner, A.; Rademann, J.; Waldmann, H. Discovery of Mycobacterium tuberculosis protein tyrosine phosphatase A (MptpA) inhibitors based on natural products and a fragment-based approach. ChemBioChem 2005, 6, 1749–1753. [Google Scholar] [CrossRef]
- Greenstein, A.E.; Grundner, C.; Echols, N.; Gay, L.M.; Lombana, T.N.; Miecskowski, C.A.; Pullen, K.E.; Sung, P.Y.; Alber, T. Structure/function studies of Ser/Thr and Tyr protein phosphorylation in Mycobacterium tuberculosis. J. Mol. Microbiol. Biotechnol. 2005, 9, 167–181. [Google Scholar] [CrossRef]
- Muller, D.; Krick, A.; Kehraus, S.; Mehner, C.; Hart, M.; Kupper, F.C.; Saxena, K.; Prinz, H.; Schwalbe, H.; Janning, P.; et al. Sponge-related cyanobacterial peptides with Mycobacterium tuberculosis protein tyrosine phosphatase inhibitory activity. J. Med. Chem. 2006, 49, 4871–4878. [Google Scholar] [CrossRef]
- Malhotra, V.; Arteaga-Cortes, L.T.; Clay, G.; Clark-Curtiss, J.E. Mycobacterium tuberculosis protein kinase K confers survival advantage during early infection in mice and regulates growth in culture and during persistent infection: Implications for immune modulation. Microbiology 2010, 156, 2829–2841. [Google Scholar] [CrossRef]
- Malhotra, V.; Okon, B.P.; Clark-Curtiss, J.E. Mycobacterium tuberculosis protein kinase K enables growth adaptation through translation control. J. Bacteriol. 2012, 194, 4184–4196. [Google Scholar]
- Strong, H.L. Substituted quinoline herbicide intermediates and process. U.S. Patent 5625068 A, 29 April 1997. [Google Scholar]
- Grossmann, K. Quinclorac belongs to a new class of highly selective auxin herbicides. Weed Sci. 1998, 46, 707–716. [Google Scholar]
- Grossmann, K.; Kwiatkowski, J.; Tresch, S. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galium. aparine L.). J. Exp. Bot. 2001, 362, 1811–1816. [Google Scholar] [CrossRef]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest. Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef]
- Gollut, J.J.R.; Gayet, A.J.A. Process for the preparation of a quinoline carboxylic acid. WO2013072376 A1, 23 May 2013. [Google Scholar]
- Musiol, R.; Jampilek, J.; Kralova, K.; Richardson, D.R.; Kalinowski, D.; Podeszwa, B.; Finster, J.; Niedbala, H.; Palka, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues. Bioorg. Med. Chem. 2007, 15, 1280–1288. [Google Scholar] [CrossRef]
- Musiol, R.; Tabak, D.; Niedbala, H.; Podeszwa, B.; Jampilek, J.; Kralova, K.; Dohnal, J.; Finster, J.; Mencel, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis inhibiting activity. Bioorg. Med. Chem. 2008, 16, 4490–4499. [Google Scholar] [CrossRef]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Photosystem II and its interaction with herbicides. In Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Delaney, J.; Clarke, E.; Hughes, D.; Rice, M. Modern agrochemical research: A missed opportunity for drug discovery? Drug Discov. Today 2006, 11, 839–845. [Google Scholar] [CrossRef]
- Duke, S.O. Herbicide and pharmaceutical relationships. Weed Sci. 2010, 58, 334–339. [Google Scholar] [CrossRef]
- Swanton, C.J.; Mashhadi, H.R.; Solomon, K.R.; Afifi, M.M.; Duke, S.O. Similarities between the discovery and regulation of pharmaceuticals and pesticides: In support of a better understanding of the risks and benefits of each. Pest. Manag. Sci. 2011, 67, 790–797. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-Inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G. Irreversible protein kinases inhibitors. Curr. Med. Chem. 2011, 18, 2981–2994. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Musumeci, F.; Radi, M.; Castagnolo, D. Curr. Med. Chem. 2011, 18, 5061–5078. [CrossRef]
- Lawrence, R.M.; Dennis, K.C.; O’Neill, P.M.; Hahn, D.U.; Roeder, M.; Struppe, C. Development of a scalable synthetic route to GSK369796 (N-tert-butyl isoquine), a novel 4-aminoquinoline antimalarial drug. Org. Process. Res. Dev. 2008, 12, 294–297. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Pesko, M.; Klimesova, V.; Waisser, K. Photosynthesis-inhibiting effects of 2-benzylsulphanylbenzimidazoles in spinach chloroplasts. Chem. Pap. 2012, 66, 795–799. [Google Scholar] [CrossRef]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Part C; Colowick, P., Kaplan, N.O., Eds.; Academic Press: London, UK, 1980; Volume 69, pp. 413–434. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Effect of 2-alkylthio-6-aminobenzothiazoles and their 6-N-substituted derivatives on photosynthesis inhibition in spinach chloroplasts. Gen. Phys. Biophys. 1993, 12, 421–427. [Google Scholar]
- Kralova, K.; Sersen, F.; Miletin, M.; Hartl, J. Inhibition of photosynthetic electron transport by some anilides of 2-alkylpyridine-4-carboxylic acids in spinach chloroplasts. Chem. Pap. 1998, 52, 52–55. [Google Scholar]
- Kralova, K.; Sersen, F.; Pesko, M.; Waisser, K.; Kubicova, L. 5-Bromo- and 3,5-dibromo-2-hydroxy-N-phenylbenzamides–inhibitors of photosynthesis. Chem. Pap. 2013. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Klimesova, V.; Waisser, K. 2-Alkylsulphanyl-4-pyridine-carbothioamides - Inhibitors of oxygen evolution in freshwater alga Chlorella vulgaris. Chem. Pap. 2011, 65, 909–912. [Google Scholar] [CrossRef]
- Servusova, B.; Eibinova, D.; Dolezal, M.; Kubicek, V.; Paterova, P.; Pesko, M.; Kralova, K. Substituted N-benzylpyrazine-2-carboxamides: Synthesis and biological evaluation. Molecules 2012, 17, 13183–13198. [Google Scholar] [CrossRef]
- Atal, N.; Saradhi, P.P.; Mohanty, P. Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: Analysis of electron transport activities and changes in fluorescence yields. Plant. Cell. Physiol. 1995, 32, 943–951. [Google Scholar]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar]
- Rath, T.; Roderfeld, M.; Blocher, S.; Rhode, A.; Basler, T.; Akineden, O.; Abdulmawjood, A.; Halwe, J.M.; Goethe, R.; Bulte, M.; et al. Presence of intestinal Mycobacterium avium subspecies paratuberculosis (MAP) DNA is not associated with altered MMP expression in ulcerative colitis. BMC Gastroenterology 2011, 11, 34–54. [Google Scholar] [CrossRef]
- Barlin, G.B.; Ireland, S.J.; Nguyen, T.M.T.; Kotecka, B.; Rieckmann, K.H. Potential antimalarials. XX. Mannich base derivatives of 2-[7-(chloroquinolin-4-ylamino and 7-bromo(and 7-trifluoromethyl)-1,5-naphthyridin-4-ylamino]-4-chloro(or 4- or 6-t-butyl or 4- or 5-fluoro)-phenols and 4(or 6)-t-butyl-2-(7-trifluoromethylquinolin-4-ylamino)phenol. Aust. J. Chem. 1997, 47, 1143–1154. [Google Scholar]
- Burckhalter, J.H.; DeWald, H.A.; Tendick, F.H. An alternate synthesis of camoquin. J. Am. Chem. Soc. 1950, 72, 1024–1025. [Google Scholar] [CrossRef]
- Francois, B. N-(4-Quinolinyl)glycine derivatives, antiinflammatory and pain killing agents. DE 1965638 A, 3 September 1970. [Google Scholar]
- Price, C.C.; Leonard, N.J.; Peel, E.W.; Reitsema, R.H. Some 4-amino-7-chloroquinoline derivatives. J. Am. Chem. Soc. 1946, 68, 1807–1808. [Google Scholar] [CrossRef]
- Masarovicova, E.; Kralova, K. Approaches to Measuring Plant Photosynthesis Activity. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Pauk, K.; Zadrazilova, I.; Imramovsky, A.; Vinsova, J.; Pokorna, M.; Masarikova, M.; Cizek, A.; Jampilek, J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. in press.
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, in press. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Otevrel, J.; Bobal, P.; Zadrazilova, I.; Govender, R.; Pesko, M.; Keltosova, S.; Koleckarova, P.; Marsalek, P.; Imramovsky, A.; Coffey, A.; et al. Antimycobacterial and Photosynthetic Electron Transport Inhibiting Activity of Ring-Substituted 4-Arylamino-7-Chloroquinolinium Chlorides. Molecules 2013, 18, 10648-10670. https://doi.org/10.3390/molecules180910648
Otevrel J, Bobal P, Zadrazilova I, Govender R, Pesko M, Keltosova S, Koleckarova P, Marsalek P, Imramovsky A, Coffey A, et al. Antimycobacterial and Photosynthetic Electron Transport Inhibiting Activity of Ring-Substituted 4-Arylamino-7-Chloroquinolinium Chlorides. Molecules. 2013; 18(9):10648-10670. https://doi.org/10.3390/molecules180910648
Chicago/Turabian StyleOtevrel, Jan, Pavel Bobal, Iveta Zadrazilova, Rodney Govender, Matus Pesko, Stanislava Keltosova, Petra Koleckarova, Petr Marsalek, Ales Imramovsky, Aidan Coffey, and et al. 2013. "Antimycobacterial and Photosynthetic Electron Transport Inhibiting Activity of Ring-Substituted 4-Arylamino-7-Chloroquinolinium Chlorides" Molecules 18, no. 9: 10648-10670. https://doi.org/10.3390/molecules180910648






