Metal Complexes of Quinolone Antibiotics and Their Applications: An Update
Abstract
:1. Introduction
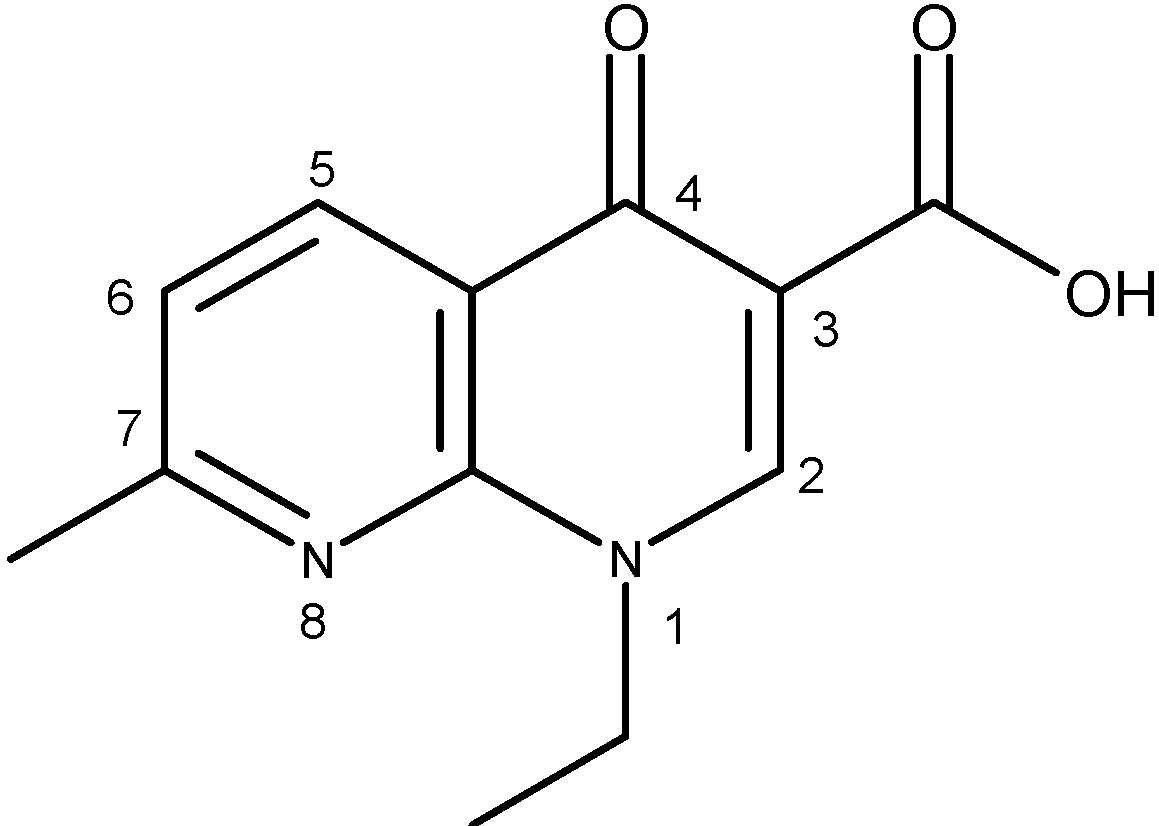

| Quinolone group/base heterocycle | X1 | X2 | X3 | R1 | R2 | R3 | R4 | Representatives | Generation |
|---|---|---|---|---|---|---|---|---|---|
| Naphthyridine (8-aza-4-quinolone) | CH | N | C | H | CH3 | C2H5 | - | Nalidixic acid | First |
| CH | N | C | F |  | C2H5 | - | Enoxacin | Second | |
| CH | N | C | F |  |  | - | Gemifloxacin | Third | |
| CH | N | C | F |  |  | - | Tosufloxacin | Third | |
| Pyridopyrimidine (6,8-diaza-4-quinolone) | CH | N | N | - |  | C2H5 | - | Pipemidic acid | First |
| CH | N | N | - |  | C2H5 | - | Piromidic acid | First | |
| Cinnoline (2-aza-4-quinolone) | N | C | C |  | C2H5 | H | Cinoxacin | First | |
| Quinoline (4-oxo-1,4-dihydroquinoline, 4-quinolone) | CH | C | C | H |  | C2H5 | H | Rosoxacin | First |
| CH | C | C |  | C2H5 | H | Oxolinic acid | First | ||
| CH | C | C | F | H |  | Flumequine | First | ||
| CH | C | C | F |  | C2H5 | H | Norfloxacin | Second | |
| CH | C | C | F |  | C2H5 | H | Pefloxacin | Second | |
| CH | C | C | F |  |  | H | Ciprofloxacin | Second | |
| CH | C | C | F |  |  | H | Enrofloxacin | Second | |
| CH | C | C | F |  | C2H5 | F | Lomefloxacin | Second | |
| CH | C | C | F |  |  | Ofloxacin | Second | ||
| CH | C | C | F |  |  | Levofloxacin | Third | ||
| CH | C | C | F |  |  | F | Sparfloxacin * | Third | |
| CH | C | C | F |  |  | OCH3 | Gatifloxacin | Third | |
| CH | C | C | F |  |  | OCH3 | Balofloxacin | Third | |
| CH | C | C | F |  |  | Cl | Clinafloxacin | Fourth | |
| CH | C | C | F |  |  | Cl | Sitafloxacin | Fourth | |
| CH | C | C | F |  |  | OCH3 | Moxifloxacin | Fourth | |
| Quinolone generation | Characteristic features |
|---|---|
| First | Active against Gram negative bacteria. |
| High protein binding. | |
| Short half life. | |
| Low serum and tissue concentrations. | |
| Uncomplicated urinary tract infection. | |
| Oral administration. | |
| Second | Class I (enoxacin, norfloxacin, lomefloxacin) |
| Enhanced activity against Gram negative bacteria. | |
| Protein binding (50%). | |
| Longer half life than the first generation. | |
| Moderate serum and tissue concentrations. | |
| Uncomplicated or complicated urinary tract infections. | |
| Oral administration. | |
| Class II (ofloxacin, ciprofloxacin) | |
| Enhanced activity against Gram negative bacteria. | |
| Atipical pathogens, Pseudomonas aeruginosa (ciprofloxacin). | |
| Protein binding (20%–50%). | |
| Moderate to long half life. | |
| Higher serum and tissue concentrations compared with class I. | |
| Complicated urinary infections, gastroenteritis, prostatitis, nosocomial infections. | |
| Oral and iv administration. | |
| Third | Active against Gram negative and Gram positive bacteria. |
| Similar pharmacokinetic profile as for second generation (class II). | |
| Similar indications and mode of administration. Consider for community aquired pneumonia in hospitalized patients. | |
| Fourth | Extended activity against Gram positive and Gram negative bacteria. |
| Active against anaerobes and atypical bacteria. | |
| Oral and i.v. administration. | |
| Consider for treatment of intraabdominal infections. |
2. Chemical Properties of Quinolones Related to Complexation Process
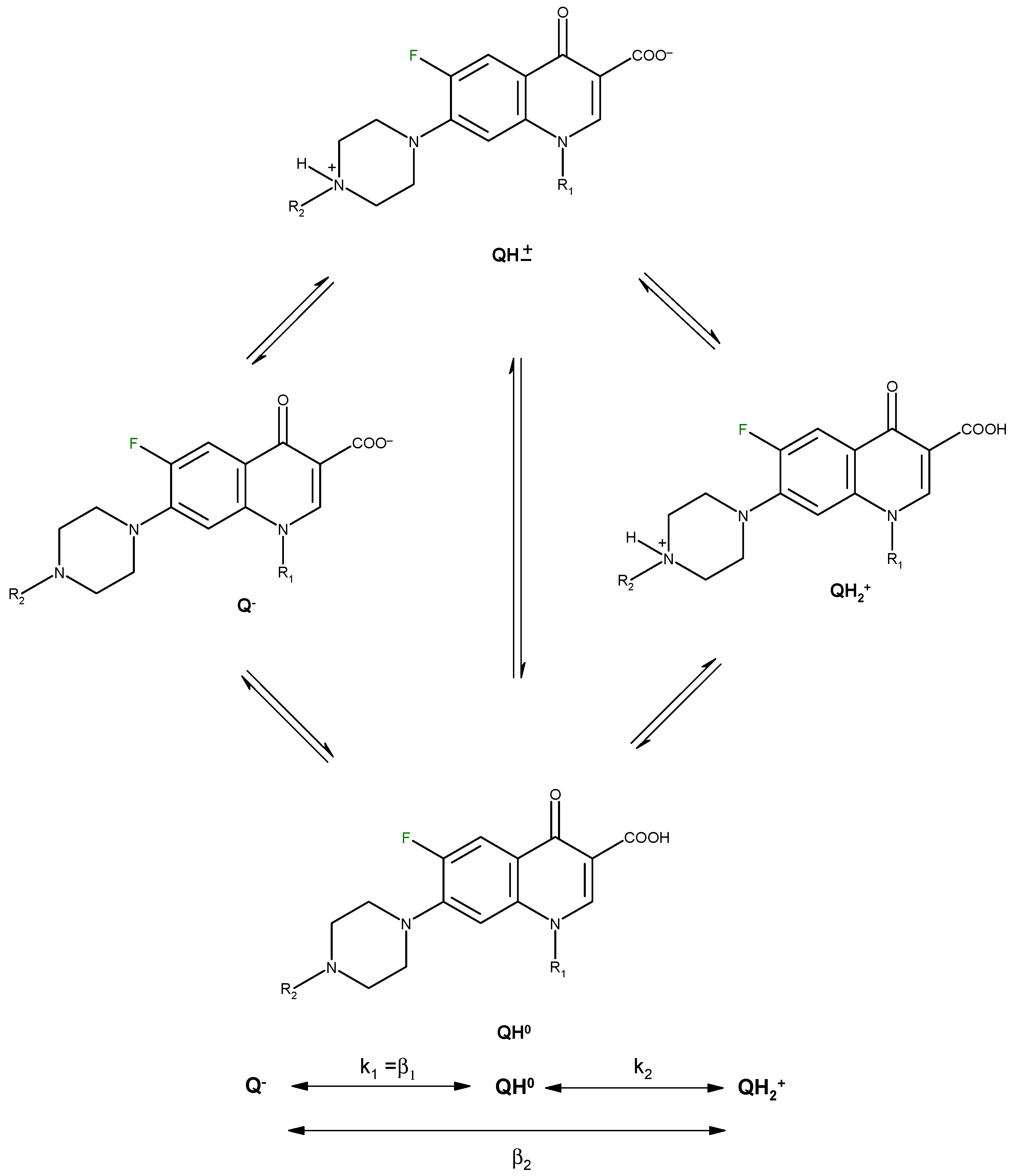
| Compound | log β1 | log β2 = log Ka2 | log β1-log β2 = log Ka1 | Isoelectric point | Reference |
|---|---|---|---|---|---|
| Norfloxacin | 14.68 | 8.38 | 6.30 | 7.34 | [19] |
| 14.73 | 8.51 | 6.22 | 7.37 | [23] | |
| Ofloxacin | 14.27 | 8.22 | 6.05 | 7.14 | [19] |
| 13.94 | 8.25 | 5.69 | 6.97 | [23] |
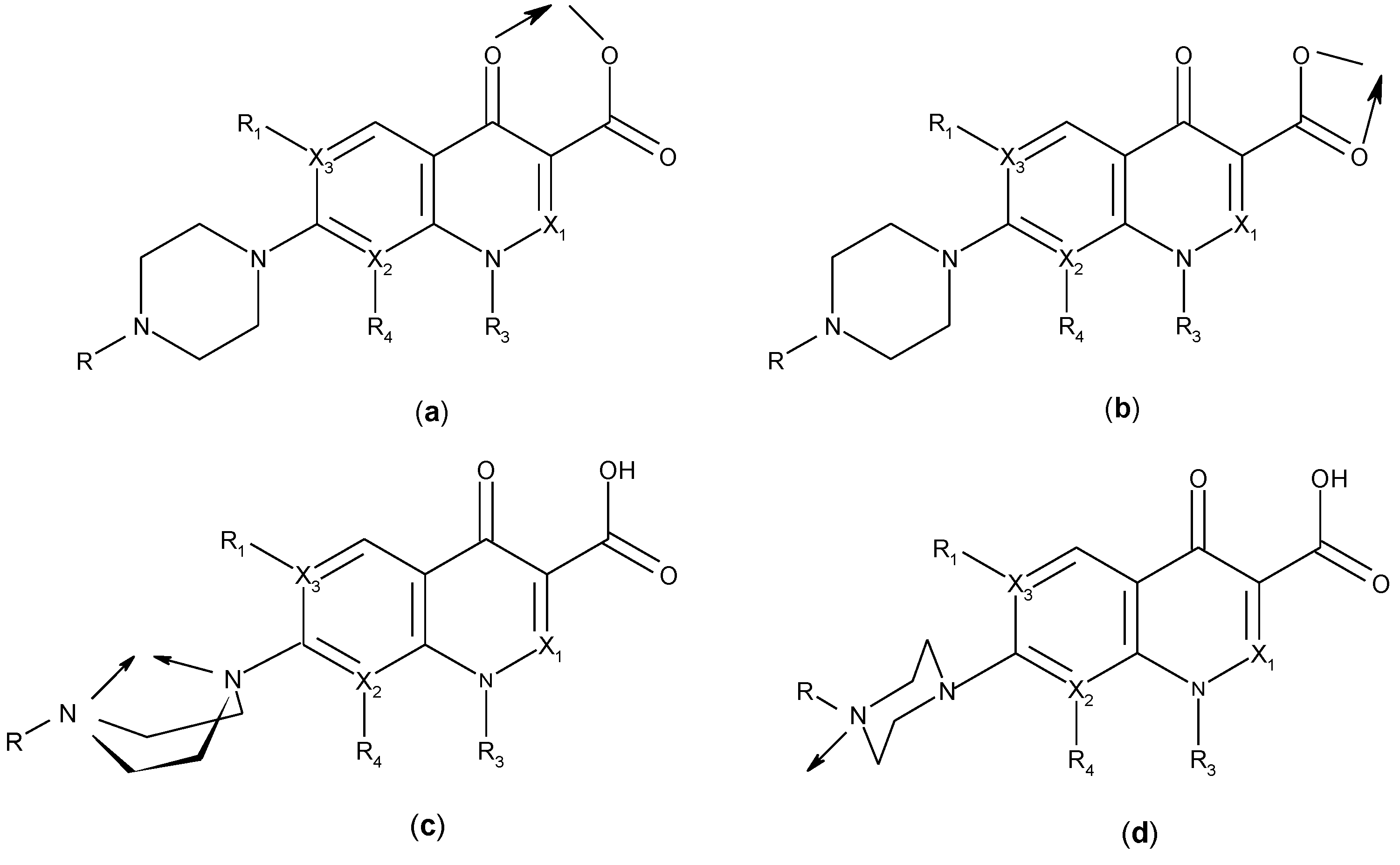
3. Metal Complexes of Quinolones
3.1. Metal-Quinolone Chelates
| Ligand | Metalion | Molar ratio M:L | General formulae of the complexes | Complex tested/investigated for | Reference |
|---|---|---|---|---|---|
| Pipemidic acid | VO2+ | 1:2 | [VO(PPA)2(H2O)] | DNA binding antimicrobial activity | [28] |
| Mn2+ | 1:2 | [Mn(PPA)2(H2O)2] | |||
| Fe3+ | 1:3 | [Fe(PPA)3] | |||
| Co2+ | 1:2 | [Co(PPA)2(H2O)2] | |||
| Ni2+ | 1:2 | [Ni(PPA)2(H2O)2] | |||
| Zn2+ | 1:2 | [Zn(PPA)2(H2O)2] | |||
| MoO22+ | 1:2 | [MoO2(PPA)2] | |||
| Cd2+ | 1:2 | [Cd(PPA)2(H2O)2] | |||
| UO22+ | 1:2 | [UO2(PPA)2] | |||
| Cu2+ | 1:2 | [Cu(PPA)2(H2O)] | DNA binding antimicrobial activity | [29] | |
| Fe3+ | 1:1 | [Fe (PPA)(HO)2(H2O)]2 | - | [30] | |
| Cinoxacin | Cu2+ | 1:2 | [Cu(Cx)2(H2O)]·3H2O | - | [31] |
| Ni2+ | [Ni(Cx)2(DMSO)2]·4H2O | ||||
| Cu2+ | 1:2 | [Cu(Cx)2]·2H2O | antimicrobial activity | [32] | |
| Co2+ | 1:3 | [Co(Cx)3]Na·10H2O | antimicrobial activity | [33] | |
| Cu2+ | 1:2 | [Cu(Cx)2]·2H2O Cu(Cx)(HCx)Cl·2H2O | |||
| Zn2+ | 1:2 | [Zn(Cx)2]·4H2O | |||
| Cd2+ | 1:1 | Cd(Cx)Cl·H2O | |||
| Cd2+ | 1:3 | Na2[(Cd(Cx)3)(Cd(Cx)3(H2O))] 12H2O | - | [34] | |
| Oxolinic acid | Cu2+ | 1:2 | [Cu(oxo)2(H2O)] | DNA binding antimicrobial activity | [35] |
| Ni2+ | 1:2 | [Ni(oxo)2(H2O)2] | DNA binding | [36] | |
| Zn2+ | 1:2 | [Zn(oxo)2(H2O)2] | DNA binding | [37] | |
| VO2+ | 1:2 | [VO(oxo)2(H2O)] | DNA binding | [38] | |
| Mn2+ | 1:2 | [Mn(oxo)2(H2O)2] | |||
| Fe3+ | 1:3 | [Fe(oxo)3] | |||
| Co2+ | 1:2 | [Co(oxo)2(H2O)2] | |||
| Ni2+ | 1:2 | [Ni(oxo)2(H2O)2] | |||
| Zn2+ | 1:2 | [Zn(oxo)2(H2O)2] | |||
| Cd2+ | 1:2 | [Cd(oxo)2(H2O)2] | |||
| MoO22+ | 1:2 | [MoO2(oxo)2] | DNA binding antimicrobial activity | [39] | |
| UO22+ | 1:2 | [UO2(oxo)2] | |||
| Flumequine | Cu2+ | 1:2 | [Cu(flmq)2(OH2)2] | - | [40] |
| Zn2+ | [Zn(flmq)2(OH2)2]·H2O | ||||
| Cu2+ | 1:2 | [Cu(flmq)2(H2O)] | DNA binding albumin binding | [41] | |
| Ni2+ | 1:2 | [Ni(flmq)2(H2O)2] | DNA binding albumin binding | [42] | |
| Zn2+ | 1:2 | [Zn(flmq)2(H2O)2] | DNA binding albumin binding | [43] |
| Ligand | Metal ion | Molar ratio M:L | General formulae of the complexes | Complex tested/ investigated for | Reference |
|---|---|---|---|---|---|
| Enoxacin | Co2+ | 1:2 | [Co(HEx)2(ClO4)2]·3H2O [Co(HEx)2(NO3)2]·2H2O | antimicrobial activity DNA oxidative cleavage | [44] |
| Cu2+ Ni2+ | 1:2 | [M(Ex)2(H2O)2]·3H2O (M = CuII, NiII or MnII) | antimicrobial activity | [45] | |
| Mn2+ Fe3+ | [Fe(Ex)(H2O)2]Cl·4H2O | antiinflammatory activity | |||
| Ni2+ | 1:2 | Ni(Ex)2·2.5H2O | DNA binding | [46] | |
| Norfloxacin | Mg2+ | 1:2 | [M(Nf)2](ClO4)2·H2O | - | [47] |
| Ca2+ | M: Mg2+, Ca2+ (n = 4), | ||||
| Ba2+ | M: Ba2+ (n = 5) | ||||
| Al3+ | 1:3 | [(Nf·HCl)3Al] | solubility behavior | [48] | |
| Bi3+ | 1:4 | [Bi (C16H18FN3O3)4(H2O)2] | antimicrobial activity solubility behavior | [49] | |
| Bi3+ | 1:3 | [Bi(C16H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Mn2+ | 1:2 | [M(Nf)2]X2·8H2O | - | [51] | |
| Co2+ | (X = CH3COO-or SO42-). | ||||
| Fe3+ | 1:3 | [Fe(Nf)3]Cl3·12H2O | - | ||
| Co2+ | 1:2 | [Co(NfH-O,O’)2(H2O)2](NO3)2 | - | [52] | |
| Mn2+ Co2+ | 1:1 1:1 | [MnCl2(Nf)(H2O)2] [CoCl2(Nf)(H2O)2] | biological evaluation against Trypanosoma cruzi | [53] | |
| Ni2+ | 1:2 | [Ni(Nf)2]·6H2O | DNA binding | [46] | |
| Cu2+ | 1:2 | Cu(HNf)2·5H2O | - | [54] | |
| [Cu(HNf)2]Cl2·2H2O | - | ||||
| Cu(HNf)2(NO3)2·H2O | - | ||||
| 1:2 | [Cu(NfH)2]Cl2·6H2O | DNA binding albumin binding | [55] | ||
| Zn2+ | 1:2 | [Zn(Nf)2]·5H2O | - | [56] | |
| Zn2+ Cd2+ Hg2+ | 1:2 | [M(Nf)2]X2·nH2O [M = Zn(II), (X = Cl−, CH3COO−, Br− and I−), Cd(II), (X = Cl−, NO3− and SO42−) and Hg(II) (X = Cl−, NO3− and CH3COO−)] | antimicrobial activity | [57] | |
| ZrO2+ UO22+ | 1:2 1:3 | [ZrO(Nf)2Cl]Cl·15H2O [UO2(Nf)3](NO3)2·4H2O | antimicrobial activity | [58] | |
| W0 | [W(H2O)(CO)3(H-Nf)]· (H-Nf)·H2O | antimicrobial activity | [59] | ||
| Ru3+ | 1:2 | [Ru(Nf)2Cl2]·4H2O | - | [60] | |
| Pt2+ | 1:2 | [Pt(Nf)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(Nf)]Cl | DNA binding albumin binding cytotoxic activitycell cycle | [62] | |
| Y3+ Pd2+ | 1:2 1:2 | [Y(Nf)2(H2O)2]Cl3·10H2O [Pd(Nf)2]Cl2·3H2O | antimicrobial activity | [63] | |
| La3+ Ce3+ | 1:3 1:3 | [La(Nf)3]·3H2O [Ce(Nf)3]·3H2O | antimicrobial activity | [64] | |
| Ln= Nd(III) Sm(III) Ho(III) | 1:4 | [N(CH3)4][Ln(Nf)4]·6H2O | interaction with DNA and albumin | [65] | |
| Pefloxacin | Bi3+ | 1:3 | [Bi(C17H19FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] |
| Zn2+ | 1:2 | [Zn (Pf)2(H2O)] ·2H2O | - | [66] | |
| Pt2+ | 1:2 | [Pt(Pf)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Ciprofloxacin | Mg2+ | 1:2 | [Mg(Cf)2]·2.5H2O | DNA binding | [67] |
| Mg2+ | 1:2 | [Mg(Cf)2(H2O)2]·2H2O | antimicrobial activity | [68] | |
| Mg2+ | 1:2 1:3 | [Mg(H2O)2(CfH)2](NO3)2·2H2O [Mg(CfH)3](SO4)·5H2O | - | [69] | |
| Mg2+ Ca2+ Ba2+ | 1:2 | [M(Cf)2](ClO4)2·H2O M: Mg2+(n = 6) M: Ca2+ (n = 4) M: Ba2+(n = 2) | - | [47] [70] | |
| Mg2+ Zn2+ Co2+ | 1:2 | [Mg(Cf)2(H2O)2]·2H2O [Zn(Cf)2]·3H2O [Co(Cf)2]·3H2O | - | [22] | |
| Al3+ | 1:3 | [(Cf·HCl)3Al] | [48] | ||
| Bi3+ | 1:3 | [Bi(C17H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| VO2+ | 1:2 | [VO(Cf)2(H2O)] | - | [71] | |
| Mn2+ Co2+ Ni2+ Cu2+ Zn2+ Cd2+ | 1:1 | [Mn(Cf)(OAc)(H2O)2]·3H2O [Co(Cf)(OAc)(H2O)2]·3H2O [Ni(Cf)(OAc)]·6H2O [Cu(Cf)(OAc)(H2O)2]·3H2O [Zn(Cf)(OAc)]·6H2O [Cd(Cf)(OAc)(H2O)2]·3H2O | antimicrobial activity | [72] | |
| Mn2+ Fe3+, Co2+ Ni2+ MoO22+ | 1:2 for M2+ 1:3 for Fe3+ | [Mn(Cf)2(H2O)2] [Fe(Cf)3] [Co(Cf)2(H2O)2] [Ni(Cf)2(H2O)2] [MoO2(Cf)2] | DNA binding | [73] | |
| Co2+ Zn2+ Cd2+ Ni2+ Cu2+ | 1:2 | [Co(Cf)2(H2O)]·9H2O [Zn(Cf)2(H2O)2]·8H2O [Cd(HCf)2(Cl)2 ]·4H2O M(Cf)2·xH2O [M = Ni, Cu, Cd] | antimicrobial activity | [34] | |
| Co2+ | 1:2 | [Co(Cf)2]·3H2O | - | [22] | |
| Cu2+ | 1:2 | [Cu(HCf)2](NO3)2]·6H2O | - | [74] | |
| Cu2+ | 1:2 | [Cu(Cf)2]Cl2·11H2O | - | [75] | |
| Cu2+ | 1:2 | [Cu(Cf)2]Cl2·6H2O | - | [76] | |
| Cu2+ | 1:2 | [Cu(HCf)2(ClO4)2]·6H2O [Cu(HCf)2(NO3)2]·6H2 | antimicrobial activity | [44] | |
| 1:1 | [Cu(HCf)(C2O4)]·2H2O | DNA oxidative cleavage | [44] | ||
| Cu2+/ Cu+ | 3:2 | [CuII(Cf)2(CuICl2)2] | antimicrobial activity Gyrase inhibition DNA cleavage | [77] | |
| Ru3+ | 1:2 | [Ru(Cf)2Cl2]Cl·3H2O | - | [60] | |
| 1:3 | [Ru(Cf)3]·4H2O | DNA interaction | [78] | ||
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Eu3+ | 1:2 | [Eu(CfH)(Cf)(H2O)4]Cl2·4.55H2O | - | [80] | |
| Lomefloxacin | Bi3+ | 1:3 | [Bi(C17H18F2N3O3)3(H2O)2] | antimicrobial activity, including H. pylori | [50] |
| Y3+ | 1:2 | [Y(LFX)2Cl2]Cl·12H2O | antimicrobial activity | [81] | |
| ZrO2+ | 1:2 | [ZrO(LFX)2Cl]Cl·15H2O | |||
| UO22+ | 1:3 | [UO2(LFX)3](NO3)2·4H2O | |||
| Cr3+ | 1:1 | [Cr(LFX)(H2O)4]Cl3 | antimicrobial, antifungal, and anticancer activity | [82] | |
| Mn2+ | 1:1 | [Mn(LFX)(H2O)4]Cl2 | |||
| Fe3+ | 1:1 | [Fe(LFX)(H2O)4]Cl3·H2O | |||
| Co2+ | 1:1 | [Co(LFX)(H2O)4]Cl2 | |||
| Ni2+ | 1:1 | [Ni(LFX)(H2O)4]Cl2·H2O | |||
| Cu2+ | 1:1 | [Cu(LFX)(H2O)4]Cl2·2H2O | |||
| Zn2+ | 1:1 | [Zn(LFX)(H2O)4]Cl2 | |||
| Th(IV) | 1:1 | [Th(LFX)(H2O)4]Cl4 | |||
| UO22+ | 1:1 | [UO2(LFX)(H2O)2](NO3)2 | |||
| Ofloxacin | Mg2+ | 1:2 | [Mg(R-oflo)(S-oflo)(H2O)2]·2H2O | antimicrobial activity | [83] |
| Ca2+ | 1:1 | Ca(oflo)Cl·2H2O | - | [84] | |
| Mg2+ | Mg(oflo)Cl·2H2O | ||||
| Ba2+ | Ba(oflo)Cl·2H2O | ||||
| Ni2+ | Ni(oflo)Cl·2H2O | ||||
| Co2+ | Co(oflo)Cl·2H2O | ||||
| Zn2+ | Zn(oflo)Cl·H2O | ||||
| Cu2+ | 1:2 | [CuII(ofloH)2][(CuICl2)2] | DNA binding albumin binding | [55] | |
| Co2+ Zn2+ | 1:2 | [M(oflo)2]·4H2O | - | [85] | |
| Cu2+ | 1:1 | M(oflo)Cl·2.5H2O | - | [86] | |
| Ni2+ | M(oflo)(SO4)0.5·2.5H2O | ||||
| M(oflo) (NO3)·2.5H2O | |||||
| 1:2 | [Cu(oflo)2·H2O]·2H2O | ||||
| Ni(oflo)2·3H2O | |||||
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(oflo)2] | DNA binding antimicrobial activity | [61] | |
| Bi3+ | 1:3 | [Bi(C17H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Pr3+ Nd3+ | 1:1 | [PrL(NO3)2(CH3OH)](NO3) [NdL(NO3)2(CH3OH)](NO3) | DNA binding DNA cleavage activity antioxidation properties | [87] | |
| Enrofloxacin | VO2+ | 1:2 | [VO(erx)2(H2O)] | antimicrobial activity DNA binding | [88] |
| MO22+ | 1:2 | [MoO2(erx)2] | antimicrobial activity DNA binding | [89] | |
| Mn2+ Fe3+ Co2+ Ni2+ Zn2+ Cd2+ UO22+ | 1:2 for M2+, 1:3 for Fe3+ | [Mn(erx)2(H2O)2] [Fe(erx)3] [Co(erx)2(H2O)2] [Ni(erx)2(H2O)2] [Zn(erx)2(H2O)2] [Cd(erx)2(H2O)2] [UO2(erx)2] | antimicrobial activity DNA binding | [90] | |
| Ni2+ | 1:2 | [Ni(erx)2(H2O)2] | DNA binding albumin binding | [91] | |
| Cu2+ | 1:2 | [Cu(erx)2]Cl | antimicrobial activity | [92] | |
| Cu2+ | 1:2 | [Cu(erx)2(H2O)] | DNA binding antimicrobial activity | [93] | |
| Cu2+ | 1:2 | [Cu(erx)2(H2O)2] | - | [94] | |
| Ru3+ | 1:2 | [Ru(erx)2Cl2]Cl·5H2O | - | [60] |
| Ligand | Metal ion | Molar ratio M:L | General formulae of the complexes | Complex tested/investigated for | Reference |
|---|---|---|---|---|---|
| Sparfloxacin | Bi3+ | 1:3 | [Bi(C19H21F2N4O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] |
| Fe3+, VO2+ Mn2+ Ni2+ UO22+ | 1:3 1:2 for M2+ | [Fe(sf)3] [VO(sf)2(H2O)] [Mn(sf)2(H2O)2] [Ni(sf)2(H2O)2] [UO2(sf)2] | DNA binding Serum albumin binding | [95] | |
| Co2+ | 1:2 | [Co(sf)2(H2O)2] | antimicrobial activity DNA binding | [96] | |
| Cu2+ | 1:2 | [Cu(sf)2] | antimicrobial activity DNA binding | [97] | |
| Mn2+ Co2+ | 1:1 1:1 | [MnCl2(sf)(H2O)2] [CoCl2(sf)(H2O)2] | biological evaluation against Trypanosoma cruzi | [53] | |
| MO22+ | 1:2 | [MoO2(sf)2] | antimicrobial activity DNA binding | [89] | |
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(sf)2] | DNA bindingDNA cleavage abilityantimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(sf)]Cl | DNA bindingalbumin bindingcytotoxic activitycell cycle | [62] | |
| Levofloxacin | Mg2+ | 1:2 | [Mg(S-oflo)2(H2O)2]·2H2O | antimicrobial activity | [83] |
| Mn2+ Co2+ Ni2+ Cu2+ Zn2+ | 1:2 | [M(levo)2(H2O)2]·nH2O (n = 2, excepting for Cu2+, n = 3) | antimicrobial activity immunomodulatory activity cytotoxicity | [98] | |
| Zn2+ | 1:2 | [Zn(levo)2(H2O)2] | DNA binding albumin binding | [99] | |
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(levo)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(levo)]Cl | DNA binding albumin binding cytotoxic activity cell cycle | [62] | |
| Gatifloxacin | Mg2+ Ca2+ Cr3+ Mn2+ Fe3+ Co2+ Ni2+ Cu2+ Zn2+ Cd2+ | 1:2 | [Mg(gat)2(H2O)2]Cl2·2H2O [Ca(gat)2(H2O)2]Cl2·2H2O [Cr(gat)2 Cl(H2O)2]Cl·2H2O [Mn (gat)2(H2O)2]·6H2O [Fe(gat)2Cl(H2O)2]Cl·2H2O [Co (gat)2(H2O)2]·4H2O [Ni (gat)2(H2O)2] Cl2·2H2O [Cu (gat)2(H2O)2]·H2O [Zn (gat)2(H2O)2]·2H2O [Cd (gat)2(H2O)2] Cl2·4H2O | antimicrobial activity antifungal activity antiiinflamatory | [100] |
| Zn2+ Ni2+ Co2+ | 1:2 | [M(gat)2(H2O)2]·4H2O | antimicrobial activity | [101] | |
| Bi3+ | 1:3 | [Bi(C19H21FN3O4)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Pd2+ | 1:1 | [PdCl2(L)] | - | [79] | |
| Pt2+ | 1:2 | [Pt(gat)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Rh3+ | 1:1 | [X]+fac-[RhCl3(L)(gat)]- where L = H2O, Dimethylsulfoxide (DMSO), Tetramethylenesulfoxide (TMSO); gat = Gatifloxacin and X = Na or [H(DMSO)2]. | antimicrobial activity | [102] | |
| Moxifloxacin | Cu2+ | 1:1 | [Cu(MOX)(H2O)2Cl]BF4 | anti-proliferative and apoptosis-inducing activity | [103] |
| Pd2+ Y3+ Ti(IV) Ce(IV) | 1:2 1:2 1:2 1:2 | [Pd(MOX)2(H2O)2]Cl2·6H2O [Y(MOX)2Cl2]Cl·12H2O [Ti(MOX)2](SO4)2·7H2O [Ce(MOX)2](SO4)2·2H2O | antimicrobial activity | [104] | |
| VO2+ Zr(IV) UO22+ | 1:2 1:2 1:3 | [VO(MOX)2H2O]SO4·11H2O [ZrO(MOX)2Cl]Cl·15H2O [UO2(MOX)3](NO3)2·3H2O | antimicrobial activity | [105] |

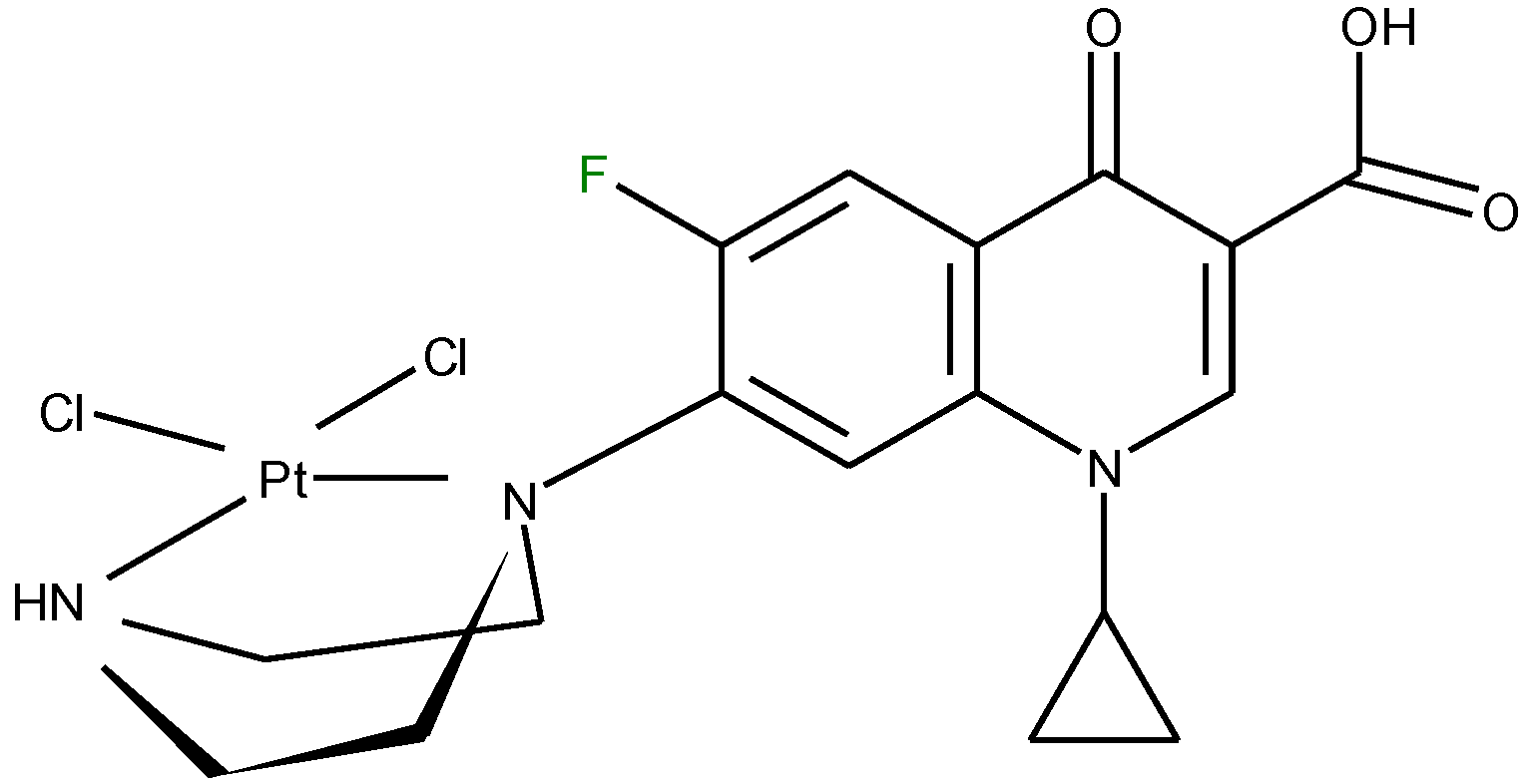
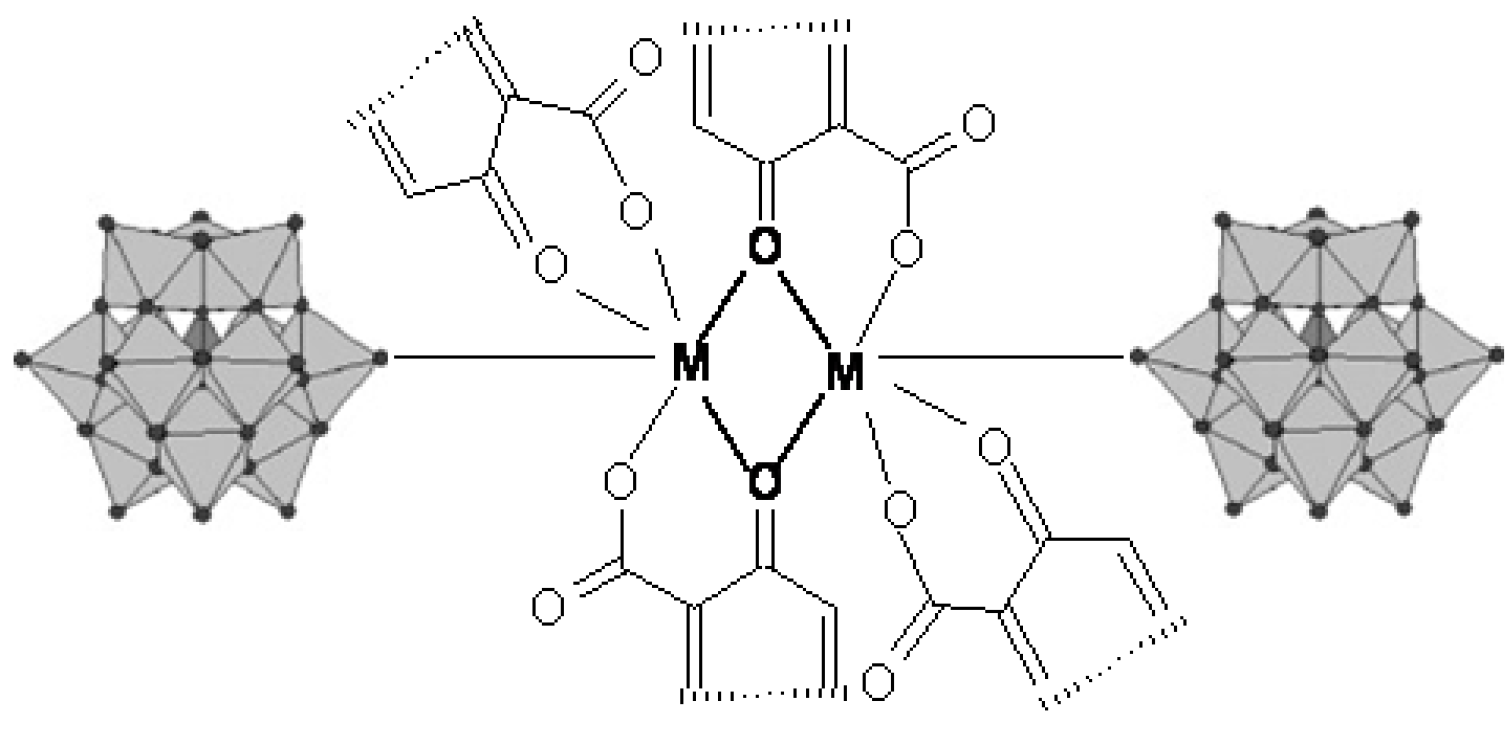
3.2. Chelates Introduced into the Polyoxometalates (POMs) Surface

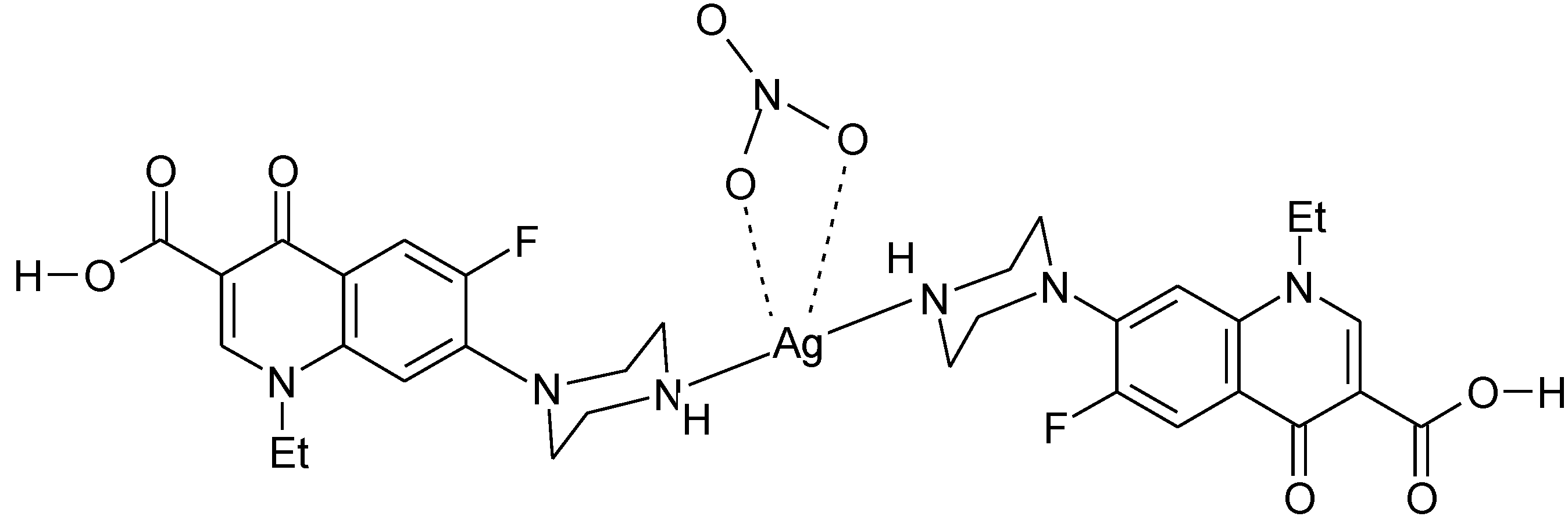
3.3. Metal Complexes with Quinolone Acting as Unidentate Ligand
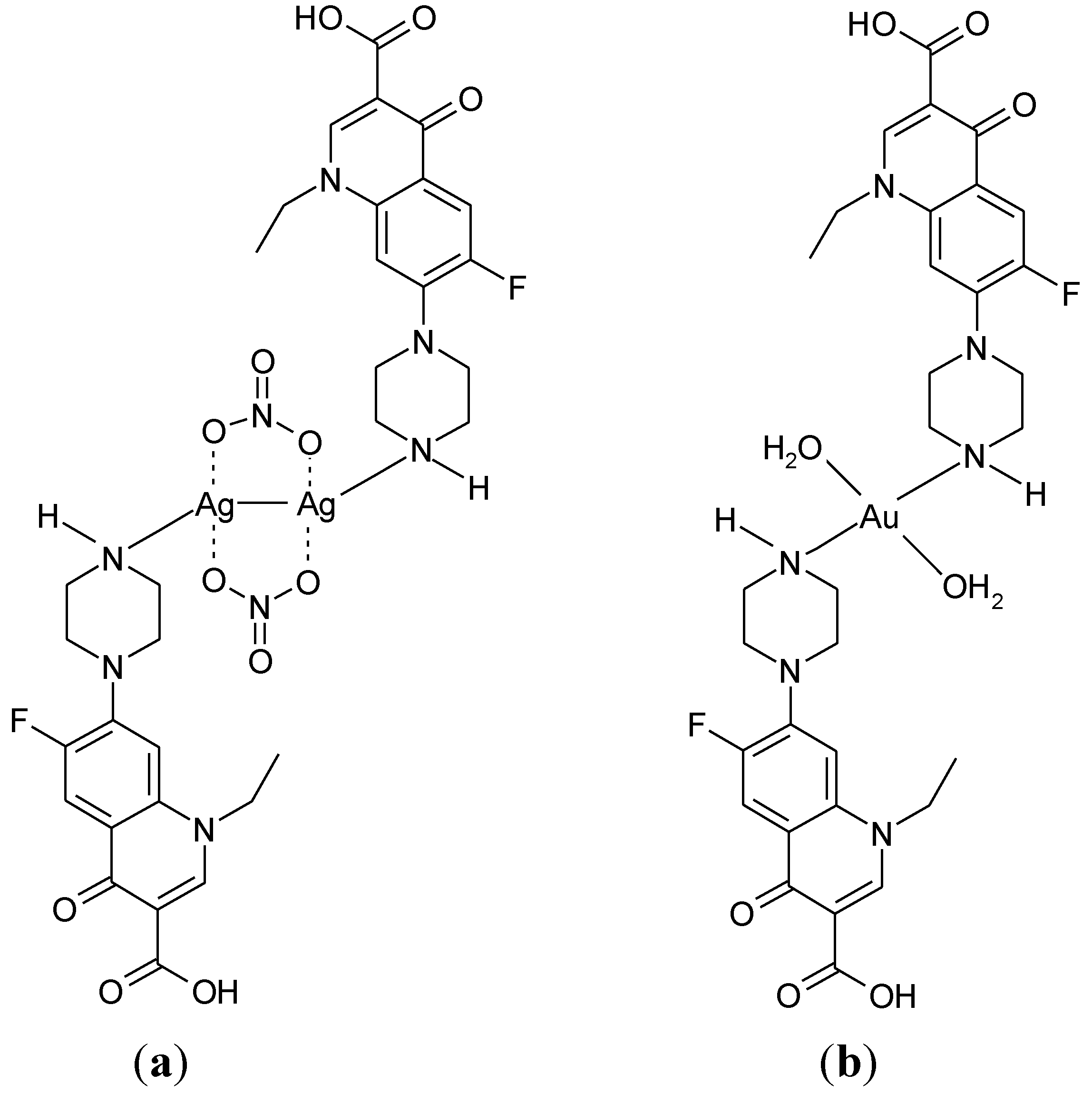
3.4. Polymeric Complexes
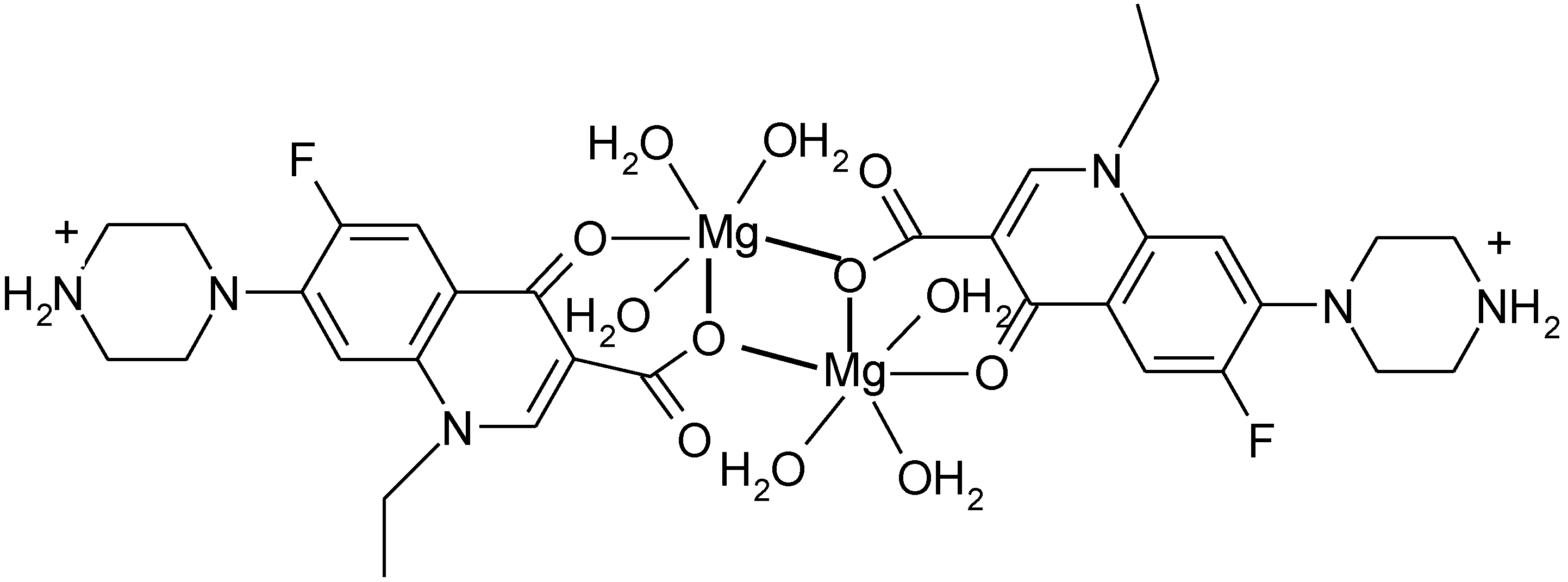
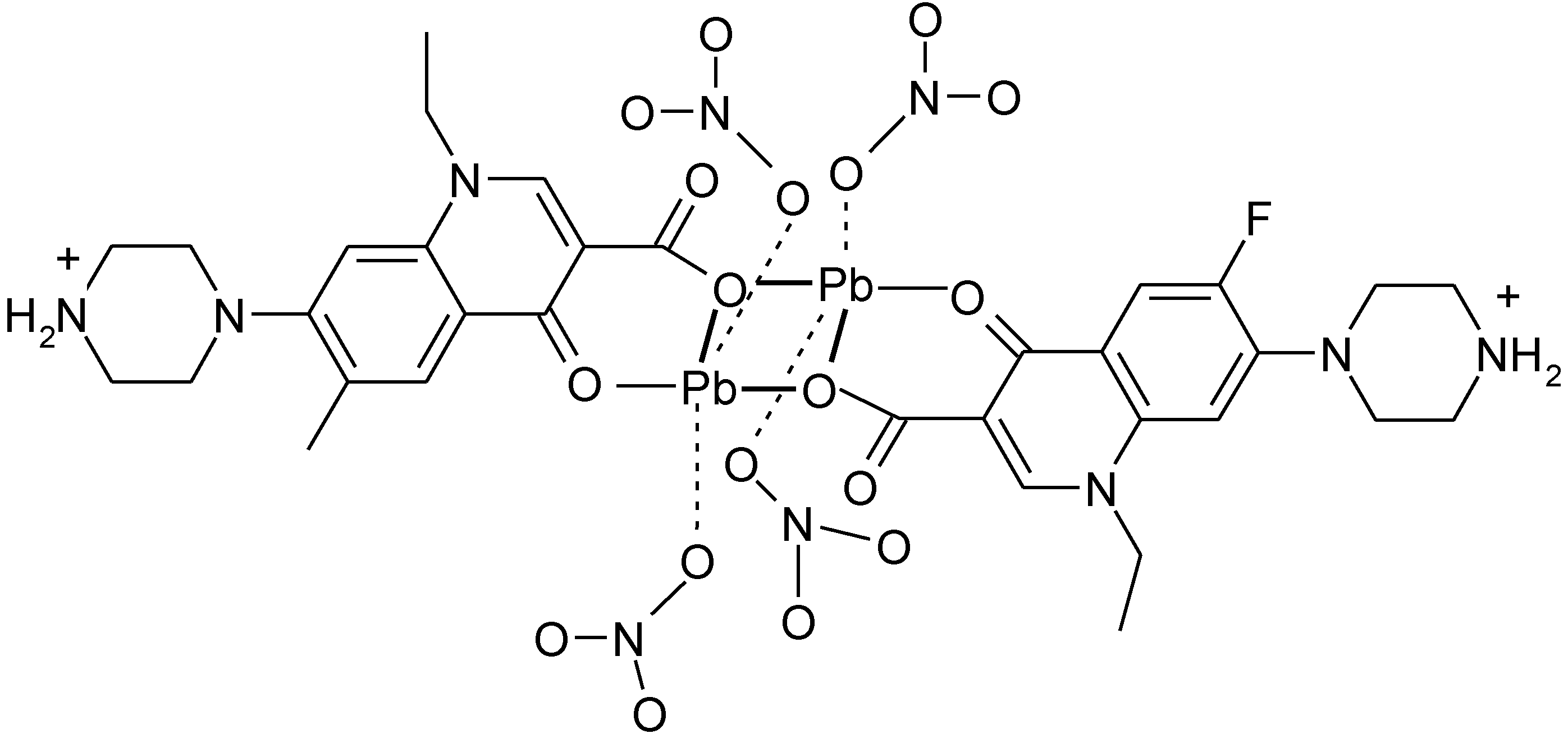

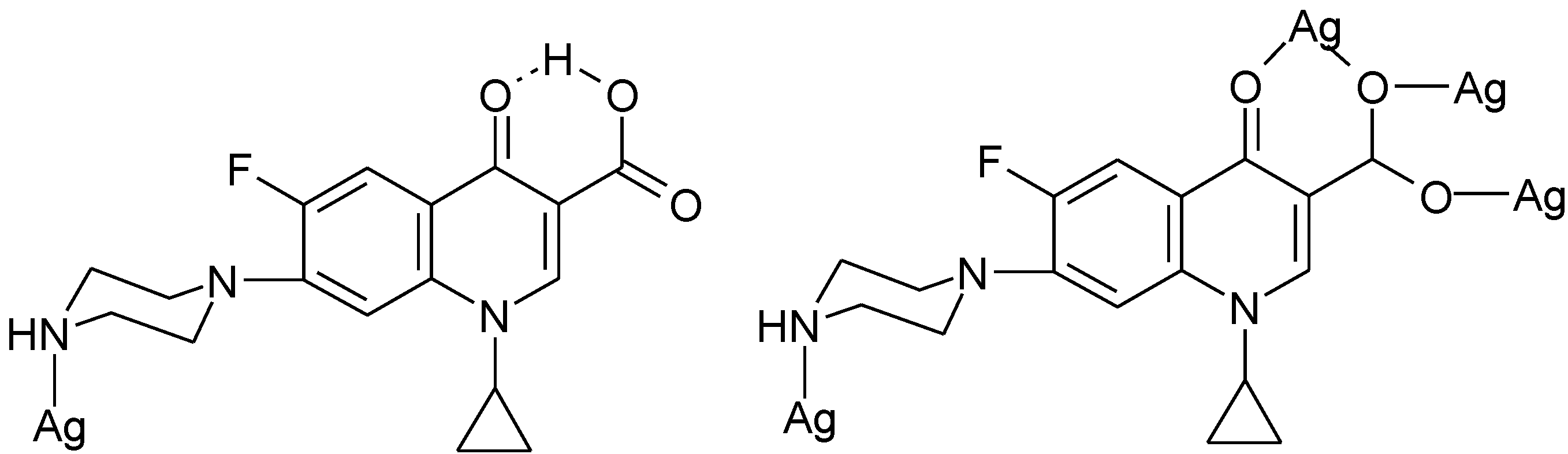
3.5. Ionic Complexes
4. Consequences and Applications of Metal-Quinolone Complexation
4.1. Pharmaceutical Aspects
4.2. Biopharmaceutical and Pharmacokinetic Implications
4.3. Mechanism of Action of Quinolones
4.4. Metal Complexes with Biological Activity
4.4.1. Antimicrobial Activity
| Compound | Bacterial strain | Ref | ||||||
|---|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (-) | |||||||
| S. aureus | B. subtilis | E. faecalis | E. coli | P. aeruginosa | K.pneumoniae | S.typhimurium | ||
| Pipemidic acid | 16.0 | - | - | 64.0 | 64.0 | - | - | [29] |
| [Cu(PPA)2(H2O)] | 16.0 | - | - | 8.0 | 8.0 | - | - | |
| [VO(PPA)2(H2O)] | 16.0 | - | - | 64.0 | 64.0 | - | - | [28] |
| [Mn(PPA)2(H2O)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [Fe(PPA)3] | 32.0 | - | - | 64.0 | 64.0 | - | - | |
| [Co(PPA)2(H2O)2] | 32.0 | - | - | 64.0 | 64.0 | - | - | |
| [Ni(PPA)2(H2O)2] | 32.0 | - | - | 64.0 | 32.0 | - | - | |
| [Zn(PPA)2(H2O)2] | 32.0 | - | - | 32.0 | 32.0 | - | - | |
| [MoO2(PPA)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [Cd(PPA)2(H2O)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [UO2(PPA)2] | 8.0 | - | - | 8.0 | 8.0 | - | - | |
| Cinoxacin | > 64 | - | > 64 | 4.0 | > 64 | 8.0 | 4.0 | [33] |
| [Cu(Cx)2]·2H 2O | > 64 | - | > 64 | 4.0 | > 64 | 8.0 | 4.0 | |
| [Co(Cx)3]Na·10H2O | > 64 | - | > 64 | 2.0 | > 64 | 2.0* | 2.0 | |
| Cu(Cx)(HCx)Cl·2H2O | > 64 | - | > 64 | 4.0 | > 64 | 8.0* | 8.0 | |
| [Zn(Cx)2]·4H2O | > 64 | - | > 64 | 4.0 | > 64 | 4.0* | 4.0 | |
| Cd(Cx)Cl·H2O | > 64 | - | 64 | 4.0 | > 64 | 8.0* | 8.0 | |
| [Cd2(Cx)4(DMSO)2]·2H2O | > 64 | - | 64 | 8.0 | > 64 | 8.0* | 8.0 | |
| [Cd2(Cx)4(H2O)2]·10H2O | > 64 | - | 64 | 4.0 | > 64 | 4.0* | 4.0 | |
| Oxolinic acid | 16 | - | - | 1 | 16 | - | - | [35] |
| [Cu(oxo)2(H2O)] | 64 | - | - | 64 | 32 | - | - | |
| Enoxacin | 1 | 0.25 | 4 | 0.12 | 0.12 | 0.12 | 0.12 | [44] |
| [Co(HEx)2(ClO4)2]·3H2O | 2 | 0.5 | 8 | 0.25 | 0.25 | 0.25 | 0.12 | |
| [Co(HEx)2(NO3)2]·2H2O | 1 | 0.25 | 8 | 0.25 | 0.25 | 0.25 | 0.12 | |
| Norfloxacin | 0.060 | - | - | 0.050 | - | 0.075 | - | [49] |
| [Bi(C16H18FN3O3)4(H2O)2] | 0.045 | - | - | 0.025 | - | 0.060 | - | |
| Ciprofloxacin | 1 | 0.12 | 1 | 0.03 | 0.5 | 0.03 | 0.016 | [44] |
| [Cu(HCf)2(NO3)2]·6H2O | 0.5 | 0.12 | 0.5 | 0.03 | 1 | 0.06 | 0.03 | |
| [Cu(HCf)(C2O4)]·2H2O | 0.5 | 0.12 | 2 | 0.06 | 1 | 0.06 | 0.06 | |
| Ciprofloxacin | 0.25 | 0.03 | 1 | 0.016 | 0.12 | 0.03 | 0.016 | [34] |
| [Co(Cf)2(H2O)]·9H2O | 0.25 | 0.06 | 1 | 0.004 | 0.12 | 0.016 | 0.008 | |
| [Zn(Cf)2(H2O)2]·8H2O | 0.25 | 0.03 | 1 | 0.004 | 0.12 | 0.03 | 0.016 | |
| Ni(Cf)2· 10H2O | 0.5 | 0.03 | 1 | 0.12 | 0.12 | 0.03 | 0.016 | |
| Cu(Cf)2· 6H2O | 0.25 | 0.03 | 1 | 0.004 | 0.12 | 0.03 | 0.008 | |
| Ofloxacin | 0.75 ** | 0.5 | 10 | 0.2 | 7 | 0.7 | 0.75 *** | [83] |
| [Mg(R-oflo)(S-oflo)(H2O)2]·2H2O | 1 ** | 0.8 | 15 | 0.25 | 10 | 1 | 1 *** | |
| Levofloxacin | 0.3 ** | 0.3 | 4 | 0.15 | 3 | 0.25 | 0.5 *** | |
| [Mg(S-oflo)2(H2O)2]·2H2O | 0.6 ** | 0.5 | 4 | 0.15 | 5 | 0.5 | 0.75 *** | |
| Enrofloxacin | 8 | - | - | 1 | 1 | - | - | [93] |
| [Cu(erx)2(H2O) | 32 | - | - | 0.125 | 0.125 | - | - | |
| erx | 0.012 | - | - | - | - | - | - | [92] |
| [Cu(erx)2]Cl | 0.0085 | - | - | - | - | - | - | |
| Herx | 8 | - | - | 1 | 1 | - | - | |
| [VO(erx)2(H2O)] | 8 | - | - | 4 | 4 | - | - | |
| [Cu(erx)2(H2O)] | 4 | - | - | 0.125 | 0.125 | - | - | |
| [MO2(erx)2] | 4 | - | - | 1 | 1 | - | - | |
| Compound | Bacterial strain | Reference | ||
|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | ||
| Norfloxacin | 12 | 25 | 13 | [63] |
| [Y(NOR)2(H2O)2]Cl3∙10H2O | 31 | 39 | 47 | |
| [Pd(NOR)2]Cl2∙3H2O | 27 | 26 | 28 | |
| [La(nor)3]∙3H2O | 12 | 10 | 9 | [64] |
| [Ce(nor)3]∙3H2O | 12 | 11 | 10 | |
4.4.2. Antifungal and Antiparasitic Activity
4.4.3. Anticancer Activity
4.5. Analytical Applications
4.5.1. Determination of Quinolones Based on Complexation with Metal Ions
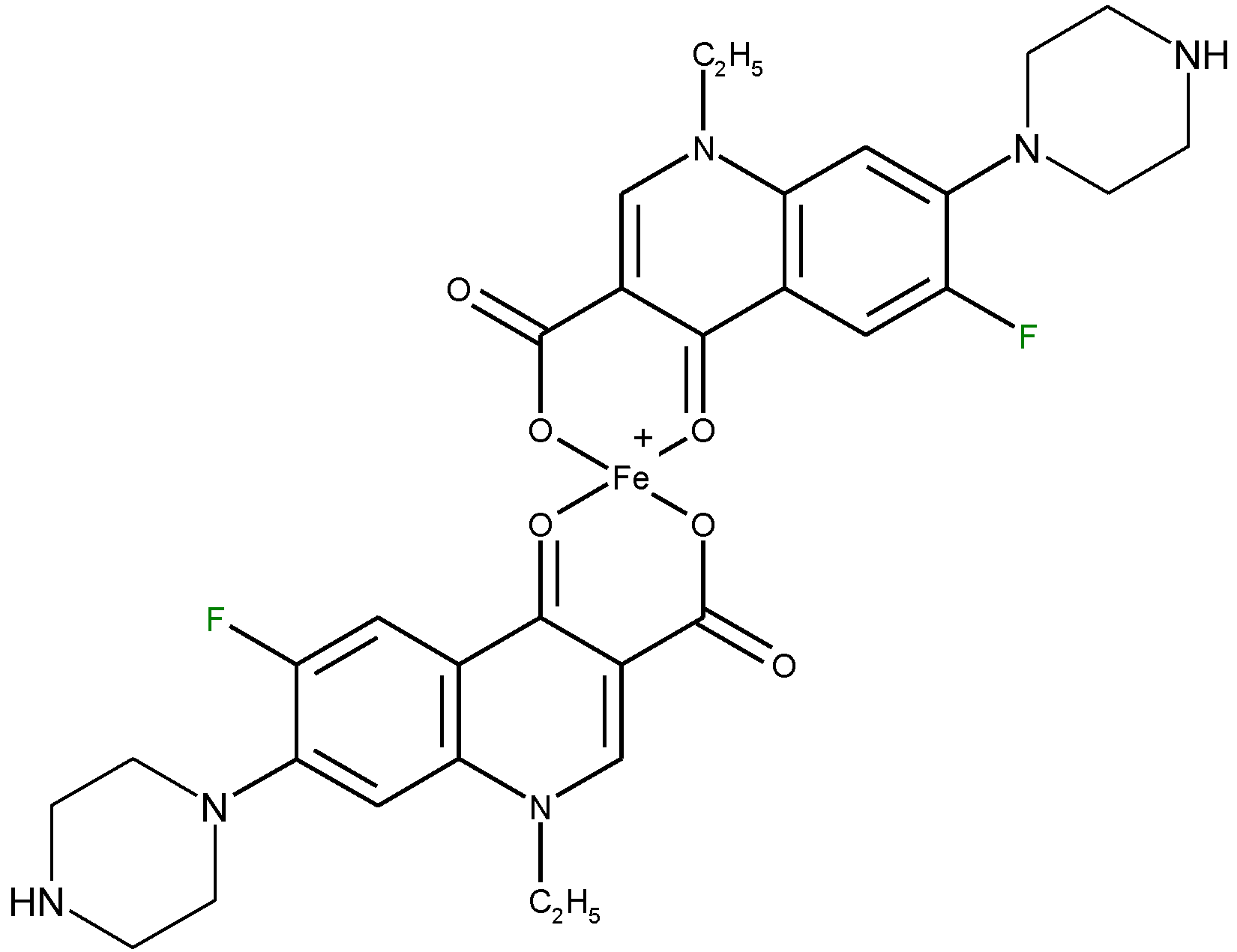
4.5.2. Determination of Metal Ions Based on Complexation with Quinolones
4.5.3. Quinolone Metal Complexes as Labels or Probes for Various Purposes
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Appelbaum, P.C.; Hunter, P.A. The fluoroquinolone antibacterials: Past, present and future perspectives. Int. J. Antimicrob. Agents 2000, 16, 5–15. [Google Scholar] [CrossRef]
- Hooper, D.C. Clinical application of quinolones. Biochim. Biophys. Acta-Gene Struct. Express. 1998, 1400, 45–61. [Google Scholar] [CrossRef]
- Buchbinder, M.; Webb, J.C.; Anderson, L.V.; McCabe, W.R. Laboratory studies and clinical pharmacology ofnalidixic acid (WIN 18, 320). Antimicrob. Agents Chemother. 1962, 2, 308–317. [Google Scholar]
- Brighty, K.E.; Gootz, T.D. Chemistry and Mechanism of Action of the Quinolone Antibacterials. In The Quinolones, 3rd ed.; Andriole, V.T., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 33–97. [Google Scholar]
- Senf, H.J. Fluorochinolone (Gyrasehemmer). Pharmazie 1988, 43, 444–447. [Google Scholar]
- Smith, J.T.; Lewin, C.S. Chemistry and Mechanisms of Action of the Quinolone Antibacterials. In The Quinolones; Andriole, V.T., Ed.; Academic Press: London, UK, 1988; pp. 23–81. [Google Scholar]
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Phys. 2002, 65, 455–464. [Google Scholar]
- King, D.E.; Malone, R.; Lilley, S.H. New classification and update on the quinolone antibiotics. Am. Fam. Phys. 2000, 61, 2741–1748. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The new fluoroquinolones: A critical review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar]
- Cozzarelli, N.R. DNA gyrase and the supercoiling of DNA. Science 1980, 207, 953–960. [Google Scholar]
- Mitscher, L.A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Maxwell, A. The molecular basis of quinolone action. J. Antimicrob. Chemother. 1992, 30, 409–414. [Google Scholar] [CrossRef]
- Schaumann, R.; Rodloff, A.C. Activities of quinolones against obligately anaerobic bacteria. Anti-Infective Agents Med. Chem. 2007, 6, 49–56. [Google Scholar] [CrossRef]
- Shen, L.L.; Chu, D.T.W. Type II DNA topoisomerases as antibacterial targets. Curr. Pharm. Des. 1996, 2, 195–208. [Google Scholar]
- Peterson, L.R. Quinolone molecular structure-activity relationships: What we have learned about improving antimicrobial activity. Clin. Infect. Dis. 2001, 33, S180–S186. [Google Scholar] [CrossRef]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. II. Acid ionization constants and their relationship to structure. Int. J. Pharmaceut. 1992, 83, 267–272. [Google Scholar] [CrossRef]
- Takacs-Novak, K.; Noszal, B.; Hermecz, I.; Kereszturi, G.; Podanyi, B.; Szasz, G. Protonation equilibria of quinolone antibacterials. J. Pharm. Sci. 1990, 79, 1023–1028. [Google Scholar] [CrossRef]
- Turel, I. The interactions of metal ions with quinolone antibacterial agents. Coord. Chem. Rev. 2002, 232, 27–47. [Google Scholar] [CrossRef]
- Zupančič, M.; Cerc Korošec, R.; Bukovec, P. The thermal-stability of ciprofloxacin complexes with magnesium (II), zinc (II) and cobalt (II). J. Therm. Anal. Calorim. 2001, 63, 787–795. [Google Scholar]
- Sasz, G.; Takacs-Novak, K.; Budvari-Barany, S.; Hermecz, J.; Jozan, M.; Lore, A.; Noszal, B. Correlation between the structures and physicochemical properties of chemoterapeutic fluoroquinolone agents. Acta Pharm. Hung. 1993, 63, 105–114. [Google Scholar]
- Turel, I.; Bukovec, N.; Farkas, E. Complex formation between some metals and a quinolone family member (ciprofloxacin). Polyhedron 1996, 15, 269–275. [Google Scholar] [CrossRef]
- Ma, H.; Chiu, F.; Li, R. Mechanistic investigation of the reduction in antimicrobial activity of ciprofloxacin by metal cations. Pharm. Res. 1997, 14, 366–370. [Google Scholar] [CrossRef]
- El-Roudi, A.M.; Soliman, E.M.; Refaiy, S.A. Effect of substituent and solvent composition on the stability of the metal complexes of 2-quinolone derivatives. Afinidad 1989, 420, 154–156. [Google Scholar]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. V. Effect of fluoroquinolones structure and pH on the complexation of various fluoroquinolones with magnesium and calcium ions. Int. J. Pharmaceut. 1993, 93, 121–129. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Katsaros, N.; Karaliota, A.; Psomas, G. Transition metal complexes with the quinolone antibacterial agent pipemidic acid: Synthesis, characterization and biological activity. Polyhedron 2007, 26, 1148–1158. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsaros, N.; Karaliota, A.; Psomas, G. Mononuclear copper(II) complexes with quinolones and nitrogen-donor heterocyclic ligands: Synthesis, characterization, biological activity and interaction with DNA. Inorg. Chim. Acta 2007, 360, 4093–4102. [Google Scholar] [CrossRef]
- Skrzypek, D.; Szymanska, B.; Kovala-Demertzi, D.; Wiecek, J.; Talik, E.; Demertzis, M.A. Synthesis and spectroscopic studies of iron (III) complex with a quinolone family member (pipemidic acid). J. Phys. Chem. Solids 2006, 67, 2550–2558. [Google Scholar] [CrossRef]
- Ruíz, M.; Ortiz, R.; Perelló, L.; Latorre, J.; Server-Carrio, J. Potentiometric and spectroscopic studies of transition-metal ions complexes with a quinolone derivative (cinoxacin). Crystal structures of new Cu (II) and Ni (II) cinoxacin complexes. J. Inorg. Biochem. 1997, 65, 87–96. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Ortiz, R.; Castineiras, A.; Maichle-Mossmer, C.; Canton, E. Synthesis, characterization and crystal structure of [Cu(Cinoxacinate)2].2H2O complex: A square planar CuO4 cromophore. Antibacterial studies. J. Inorg. Biohem. 1995, 59, 801–810. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Server-Carrio, J.; Ortiz, R.; Garcia-Granda, S.; Diaz, M.R.; Canton, E. Cinoxacin complexes with divalent metal ions. Spectroscopic characterization. Crystal structure of a new dinuclear Cd (II) complex having two chelate-bridging carboxylate groups. Antibacterial studies. J. Inorg. Biochem. 1998, 69, 231–239. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Ortiz, R.; Perelló, L.; Latorre, J.; Liu-González, M.; García-Granda, S.; Pérez-Priede, M.; Canton, E. Interaction of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin). Spectroscopic and X-ray structural characterization. Antibacterial studies. J. Inorg. Biochem. 2002, 92, 65–74. [Google Scholar] [CrossRef]
- Psomas, G.; Tarushi, A.; Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Katsaros, N. Synthesis, structure and biological activity of copper(II) complexes with oxolinic acid. J. Inorg. Biochem. 2006, 100, 1764–1773. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction. Part 2 – Interaction of nickel(II) with the antibacterial drug oxolinic acid. J. Inorg. Biochem. 2010, 104, 161–170. [Google Scholar] [CrossRef]
- Tarushi, A.; Psomas, G.; Raptopoulou, C.P.; Kessissoglou, D.P. Zinc complexes of the antibacterialdrug oxolinic acid: Structure and DNA-binding properties. J. Inorg. Biochem. 2009, 103, 898–905. [Google Scholar] [CrossRef]
- Tarushi, A.; Christofis, P.; Psomas, G. Synthesis, characterization and interaction with DNA of mononuclear metal complexes with oxolinic acid. Polyhedron 2007, 26, 3963–3972. [Google Scholar] [CrossRef]
- Tarushi, A.; Efthimiadou, E.K.; Christofis, P.; Psomas, G. Neutral mononuclear dioxomolybdenum(VI) and dioxouranium(VI)complexes of oxolinic acid: Characterization and biological evaluation. Inorg. Chim. Acta 2007, 360, 3978–3986. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Boudesocque, S.; Sayen, S.; Guillon, E. Cu(II) and Zn(II) complexes with a fluoroquinolone antibiotic: Spectroscopic and X-ray absorption characterization. Polyhedron 2011, 30, 438–443. [Google Scholar] [CrossRef]
- Chalkidou, E.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Copper(II) complexes with antimicrobial drug flumequine: Structure and biological evaluation. J. Inorg. Biochem. 2012, 113, 55–65. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction Part 3 — Nickel(II) complexes of the antibacterial drug flumequine. J. Inorg. Biochem. 2010, 104, 740–749. [Google Scholar] [CrossRef]
- Tarushi, A.; Kljun, J.; Turel, I.; Pantazaki, A.A.; Psomas, G.; Kessissoglou, D.P. Zinc(II) complexes with the quinolone antibacterial drug flumequine: Structure, DNA- and albumin-binding. New J. Chem. 2013, 37, 342–355. [Google Scholar] [CrossRef]
- Jiménez-Garrido, N.; Perelló, L.; Ortiz, R.; Alzuet, G.; González-Álvarez, M.; Cantón, E.; Liu-González, M.; García Granda, S.; Pérez-Priede, M. Antibacterial studies, DNA oxidative cleavage, and crystal structures of Cu(II) and Co(II) complexes with two quinolone family members, ciprofloxacin and enoxacin. J. Inorg. Biochem. 2005, 99, 677–689. [Google Scholar] [CrossRef]
- Arayne, S.; Sultana, N.; Haroon, U.; Mesaik, M.A. Synthesis, characterization, antibacterial and anti-inflammatory activities of enoxacin metal complexes. Bioinorg. Chem. Appl. 2009. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Li, X.; Qiu, H.-B.; Zhang, Y.-H.; Yan, H. Nickel complexes of the different quinolone antibacterial drugs: Synthesis, structure and interaction with DNA. Inorg. Chim. Acta 2012, 383, 178–184. [Google Scholar] [CrossRef]
- Al-Mustafa, J. Magnesium, calcium and barium perchlorate complexes of ciprofloxacin and norfloxacin. Acta Chim. Slov. 2002, 49, 457–466. [Google Scholar]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharmaceut. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Yadav, M.R. Bismuth-norfloxacin complex: Synthesis, physicochemical and antimicrobial evaluation. Int. J. Pharmaceut. 2007, 332, 24–30. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Megraud, F.; Yadav, M.R. Metalloantibiotics: Synthesis, characterization and antimicrobial evaluation of bismuth-fluoroquinolone complexes against Helicobacter Pylori. Acta Pharm. 2009, 59, 259–271. [Google Scholar] [CrossRef]
- Sadeek, S.A. Synthesis, thermogravimetric analysis, infrared, electronic and mass spectra of Mn(II), Co(II) and Fe(III) norfloxacin complexes. J. Mol. Struct. 2005, 753, 1–12. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Kirik, S.D.; Golovneva, I.I. Synthesis of norfloxacin compounds with cobalt(II), zinc(II), cadmium(II), and mercury(II). Russ. J. Inorg. Chem. 2009, 54, 223–225. [Google Scholar] [CrossRef]
- Batista, D.G.J.; da Silva, P.B.; Stivanin, L.; Lachter, D.R.; Silva, R.S.; Felcman, J.; Louro, S.R.W.; Teixeira, L.R.; de Nazare C. Soeiro, M. Co(II), Mn(II) and Cu(II) complexes of fluoroquinolones: Synthesis, spectroscopical studies and biological evaluation against Trypanosoma cruzi. Polyhedron 2011, 30, 1718–1725. [Google Scholar] [CrossRef]
- Ruíz, P.; Ortiz, R.; Perelló, L.; Alzuet, G.; González-Álvarez, M.; Liu-González, M.; Sanz-Ruíz, F. Synthesis, structure, and nuclease properties of several binary and ternary complexes of copper(II) with norfloxacin and 1,10 phenantroline. J. Inorg. Biochem. 2007, 101, 831–840. [Google Scholar] [CrossRef]
- Živec, P.; Perdih, F.; Turel, I.; Giester, G.; Psomas, G. Different types of copper complexes with the quinolone antimicrobial drugs ofloxacin and norfloxacin: Structure, DNA- and albumin-binding. J. Inorg. Biochem. 2012, 117, 35–47. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ge, L.G.; Zhang, X.L.; Dai, Y.J.; Chen, H.L.; Mo, L.P. Thermal decomposition kinetics of the Zn(II) complex with norfloxacin in static air atmosphere. J. Therm Anal. Calorim. 1999, 58, 269–278. [Google Scholar] [CrossRef]
- Refat, M.S.; Mohamed, G.G.; de Farias, R.F.; Powell, A.K.; El-Garib, M.S.; El-Korashy, S.A.; Hussien, M.A. Spectroscopic, thermal and kinetic studies of coordination compounds of Zn(II), Cd(II) and Hg(II) with norfloxacin. J. Therm. Anal. Calorim. 2010, 102, 225–232. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Did Amony, A.M.; El-Shwiniy, W.H.; Zordok, W.A. Uranium (VI) and zirconium (IV) of the second -generation quinolone antimicrobial drug norfloxacin: Structure and biological activity. J. Argent. Chem. Soc. 2009, 97, 51–76. [Google Scholar]
- Chen, X.-B.; Ye, Q.; Wu, Q.; Song, Y.-M.; Xiong, R.-G.; You, X.-Z. The first organometallic carbonyl tungsten complex of antibacterial drug norfloxacin. Inorg. Chem. Commun. 2004, 7, 1302–1305. [Google Scholar] [CrossRef]
- Uivarosi, V.; Badea, M.; Olar, R.; Marinescu, D.; Nicolescu, T.O.; Nitulescu, G.M. Thermal degradation behavior of some ruthenium complexes with fluoroquinolone derivatives as potential antitumor agents. J. Therm. Anal. Calorim. 2011, 105, 645–650. [Google Scholar] [CrossRef]
- Patel, M.N.; Gandhi, D.S.; Parmar, P.A. DNA interaction and in-vitro antibacterial studies of fluoroquinolone based platinum(II) complexes. Inorg. Chem. Commun. 2012, 15, 248–251. [Google Scholar] [CrossRef]
- Gouvea, L.R.; Garcia, L.S.; Lachter, D.R.; Nunes, P.R.; de Castro Pereira, F.; Silveira-Lacerda, E.P.; Louro, S.R.W.; Barbeira, P.J.S.; Teixeira, L.R. Atypical fluoroquinolone gold(III) chelates as potential anticancer agents: Relevance of DNA and protein interactions for their mechanism of action. Eur. J. Med. Chem. 2012, 55, 67–73. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; Zordok, W.A.; El-Didamony, A.M. Synthesis, spectroscopic, thermal and biological activity investigation of new Y(ΙΙΙ) and Pd(ΙΙ) norfloxacin complexes. J. Argent. Chem. Soc. 2009, 97, 128–148. [Google Scholar]
- Refat, M.S.; El-Hawary, W.F.; Mohamed, M.A. Study of the chemical chelates and anti-microbial effect of some metal ions in nanostructural form on the efficiency of antibiotic therapy “norfloxacin drug”. J. Mol. Struct. 2012, 1013, 45–54. [Google Scholar]
- Li, S.; Wang, Y.; Lin, Q.; Liu, W.; Ding, J.; Wang, Y. Synthesis, crystal structures of novel complexes of rare earth with norfloxacin, interaction with DNA and BSA. J. Rare Earths 2012, 30, 460–466. [Google Scholar] [CrossRef]
- Qi, W.; Huang, J.; An, Z. Aquabis[1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato]zinc(II) dihydrate. Acta Crystallogr. 2008, 64, m302. [Google Scholar]
- Drevenšek, P.; Poklar Ulrih, N.; Majerle, A.; Turel, I. Synthesis, characterization and DNA binding of magnesium–ciprofloxacin (cfH) complex [Mg(cf)2]·2.5H2O. J. Inorg. Biochem. 2006, 100, 1705–1713. [Google Scholar] [CrossRef]
- Turel, I.; Šonc, A.; Zupančič, M.; Sepčić, K.; Turk, T. Biological activity of some magnesium(II) complexes of quinolones. Met. Based Drugs 2000, 7, 101–104. [Google Scholar] [CrossRef]
- Turel, I.; Živec, P.; Pevec, A.; Tempelaar, S.; Psomas, G. Compounds of antibacterial agent ciprofloxacin and magnesium – crystal structures and molecular modeling calculations. Eur. J. Inorg. Chem. 2008, 23, 3718–3727. [Google Scholar]
- Al-Mustafa, J.; Taha, Z.A. Thermodynamics of the complexation of ciprofloxacin with calcium and magnesium perchlorate. Thermochim. Acta 2011, 521, 9–13. [Google Scholar] [CrossRef]
- Turel, I.; Golobič, A.; Klavžar, A.; Pihlar, B.; Buglyó, P.; Tolis, E.; Rehder, D.; Sepčić, K. Interactions of oxovanadium(IV) and the quinolone family member—ciprofloxacin. J. Inorg. Biochem. 2003, 95, 199–207. [Google Scholar] [CrossRef]
- Anacona, J.R.; Toledo, C. Synthesis and antibacterial activity of metal complexes of ciprofloxacin. Trans. Met. Chem. 2001, 26, 228–231. [Google Scholar] [CrossRef]
- Psomas, G. Mononuclear metal complexes with ciprofloxacin: Synthesis, characterization and DNA-binding properties. J. Inorg. Biochem. 2008, 102, 1798–1811. [Google Scholar] [CrossRef]
- Hernandez-Gil, J.; Perello, L.; Ortiz, R.; Alzuet, G.; Gonzalez-Alvarez, M.; Liu-Gonzalez, M. Synthesis, structure and biological properties of several binary and ternary complexes of copper(II) with ciprofloxacin and 1,10 phenanthroline. Polyhedron 2009, 28, 138–144. [Google Scholar] [CrossRef]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W.; Duckworth, P.A. Copper (II) complexes of the fluoroquinolone antimicrobial ciprofloxacin: Synthesis, X-ray structural characterization and potentiometric study. J. Inorg. Biochem. 1996, 62, 1–16. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Synthesis, characterization, and crystal structure of a copper(II) complex with quinolone family member (ciprofloxacin): Bis(1)-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-piperazin-1ylquinoline-3-carboxylate) copper(II) chloride hexahydrate. J. Inorg. Biochem. 1994, 56, 273–282. [Google Scholar] [CrossRef]
- Drevenšek, P.; Zupančič, T.; Pihlar, B.; Jerala, R.; Kolitsch, U.; Plaper, A.; Turel, I. Mixed-valence Cu(II)/Cu(I) complex of quinolone ciprofloxacin isolated by a hydrothermal reaction in the presence of L-histidine: Comparison of biological activities of various copper–ciprofloxacin compounds. J. Inorg. Biochem. 2005, 99, 432–442. [Google Scholar] [CrossRef]
- Tanimoto, M.; Dias, K.; Dovidauskas, S.; Nikolaou, S. Tuning the reaction products of ruthenium and ciprofloxacin for studies of DNA interactions. J. Coord Chem. 2012, 65, 1504–1517. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; de Almeida, M.V.; Lourenço, M.C.S.; Bezerra, F.A.F.M.; Fontes, A.P.S. Synthesis and antitubercular activity of palladium and platinum complexes with fluoroquinolones. Eur. J. Med. Chem. 2009, 44, 4107–4111. [Google Scholar] [CrossRef]
- Čurman, D.; Živec, P.; Leban, I.; Turel, I.; Polishchuk, A.; Klika, K.D.; Karaseva, E.; Karasev, V. Spectral properties of Eu(III) compound with antibacterial agent ciprofloxacin (cfqH). Crystal structure of [Eu(cfqH)(cfq)(H2O)4]Cl2· 4.55H2O. Polyhedron 2008, 27, 1489–1496. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H. Preparation, structure and microbial evaluation of metal complexes of the second generation quinolone antibacterial drug lomefloxacin. J. Mol. Struct. 2010, 98, 130–138. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational behaviour of lomefloxacin drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO2(VI) ions: Synthesis, structural characterization and biological activity studies. Spectrochim. Acta A 2011, 82, 8–19. [Google Scholar] [CrossRef]
- Drevenšek, P.; Košmrlj, J.; Giester, G.; Skauge, T.; Sletten, E.; Sepčić, K.; Turel, I. X-ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones – racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. [Google Scholar] [CrossRef]
- Sagdinc, S.; Bayari, S. Spectroscopic studies on the interaction of ofloxacin with metals. J. Mol. Struct. 2004, 691, 107–113. [Google Scholar] [CrossRef]
- Macias, B.; Villa, M.V.; Sastre, M.; Castiñeiras, A.; Borras, J. Complexes of Co(II) and Zn(II) with ofloxacin. Crystal structure of [Co(oflo)2(MeOH)2]·4MeOH. J. Pharm. Sci. 2002, 91, 2416–2423. [Google Scholar] [CrossRef]
- Macias, B.; Villa, M.; Rubio, I.; Castineiras, A.; Borras, J. Complexes of Ni (II) and Cu (II) with ofloxacin. Crystal structure of a new Cu (II) ofloxacin complex. J. Inorg. Biochem. 2001, 84, 163–170. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.-C.; Xu, Z.-H.; Zeng, Z.-Z. Crystal structure, biological studies of water-soluble rare earth metal complexes with an ofloxacin derivative. Inorg. Chim. Acta 2012, 384, 324–332. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsaros, N.; Karaliota, A.; Psomas, G. Synthesis, characterization, antibacterial activity, and interaction with DNA of the vanadyl-enrofloxacin complex. Bioorg. Med. Chem. Lett. 2007, 17, 1238–1242. [Google Scholar]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear dioxomolybdenum(VI) complexes with the quinolones enrofloxacin and sparfloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 349–356. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear metal complexes of the second-generation quinolone antibacterial agent enrofloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 1729–1738. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction. Part 4 — Structure and biological evaluation of nickel(II)–enrofloxacin complexes compared to zinc(II) analogues. J. Inorg. Biochem. 2011, 105, 63–74. [Google Scholar] [CrossRef]
- Saraiva, R.; Lopes, S.; Ferreira, M.; Novais, F.; Pereira, E.; Feio, M.J.; Gameiro, P. Solution and biological behaviour of enrofloxacin metalloantibiotics: A route to counteract bacterial resistance? J. Inorg. Biochem. 2010, 104, 843–850. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Katsarou, M.; Raptopoulou, C.P.; Karaliota, A.; Katsaros, N.; Psomas, G. Neutral and cationic mononuclear copper(II) complexes with enrofloxacin: Structure and biological activity. J. Inorg. Biochem. 2006, 100, 1378–1388. [Google Scholar] [CrossRef]
- Ftouni, H.; Sayen, S.; Boudesocque, S.; Dechamps-Olivier, I.; Guillon, E. Structural study of the copper(II)-enrofloxacin metallo–antibiotic. Inorg. Chim. Acta 2012, 382, 186–190. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Metal complexes of the third-generation quinolone antimicrobial drug sparfloxacin: Structure and biological evaluation. J. Inorg. Biochem. 2010, 104, 455–466. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Structure, antimicrobial activity and DNA-binding properties of the cobalt(II)–sparfloxacin complex. Bioorg. Med. Chem. Lett. 2008, 18, 4033–4037. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Karaliota, A.; Katsaros, N.; Psomas, G. Crystal structure, spectroscopic, and biological study of the copper(II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg. Med. Chem. Lett. 2006, 16, 3864–3867. [Google Scholar]
- Sultana, N.; Arayne, M.S.; Rizvi, S.B.S.; Haroon, U.; Mesaik, M.A. Synthesis, spectroscopic, and biological evaluation of some levofloxacin metal complexes. Med. Chem. Res. 2013, 22, 1371–1377. [Google Scholar]
- Tarushi, A.; Polatoglou, E.; Kljun, J.; Turel, I.; Psomas, G.; Kessissoglou, D.P. Interaction of Zn(II) with quinolone drugs: Structure and biological evaluation. Dalton Trans. 2011, 40, 9461–9473. [Google Scholar] [CrossRef]
- Sultana, N.; Naz, A.; Arayne, M.S.; Ahmed Mesaik, M. Synthesis, characterization, antibacterial, antifungal and immunomodulating activities of gatifloxacin–metal complexes. J. Mol. Struct. 2010, 969, 17–24. [Google Scholar]
- Li, Z.-Q.; Wu, F.-J.; Gong, Y.; Hu, C.-W.; Zhang, Y.-H.; Gan, M.-Y. Synthesis, characterization and activity against Staphylococcus of metal(II)-gatifloxacin complexes. Chin. J. Chem. 2007, 25, 1809–1814. [Google Scholar] [CrossRef]
- Mehrotra, R.; Shukla, S.N.; Gaur, P.; Dubey, A. Identification of pharmacophore in bioactive metal complexes: Synthesis, spectroscopic characterization and application. Eur. J. Med. Chem. 2012, 50, 149–153. [Google Scholar] [CrossRef]
- Patitungkho, S.; Adsule, S.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F.H. Synthesis, characterization and anti-tumor activity of moxifloxacin–copper complexes against breast cancer cell lines. Bioorg. Med. Chem. Lett. 2011, 21, 1802–1806. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; El-Attar, M.S. Synthesis, characterization and antimicrobial investigation of some moxifloxacin metal complexes. Spectrochim. Acta Part A 2011, 84, 99–110. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; Zordok, W.A.; Kotb, E. Spectroscopic studies, thermal analyses and biological evaluation of new V(IV), Zr(IV) and U(VI) moxifloxacin complexes. J. Mol. Struct. 2011, 1006, 192–209. [Google Scholar] [CrossRef]
- Serafin, A.; Stanczak, A. The complexes of metal ions with fluoroquinolones. Russ. J. Coord. Chem. 2009, 35, 81–95. [Google Scholar] [CrossRef]
- Psomas, G.; Kessissoglou, D.P. Quinolones and non-steroidal antiinflammatory drugs interacting with copper(II), nickel(II), cobalt(II) and zinc(II): Structural features, biological evaluation and perspectives. Dalton Trans. 2013, 42, 6252–6276. [Google Scholar] [CrossRef]
- Gao, F.; Yang, P.; Xie, J.; Wang, H. Synthesis, characterization and antibacterial activity of novel Fe(III), Co(II), and Zn(II) complexes with norfloxacin. J. Inorg. Biochem. 1995, 60, 61–67. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; de Almeida, M.V.; de Abreu, H.A.; Duarte, H.A.; Grazul, R.M.; Fontes, A.P.S. Platinum(II) complexes with fluoroquinolones: Synthesis and characterization of unusual metal–piperazine chelates. Inorg. Chim. Acta 2009, 362, 2060–2064. [Google Scholar] [CrossRef]
- Rusu, A.; Tóth, G.; Szőcs, L.; Kökösi, J.; Kraszni, M.; Gyéresi, A.; Noszál, B. Triprotic site-specific acid-base equilibria and related properties of fluoroquinolone antibacterials. J. Pharm. Biomed. Anal. 2012, 66, 50–57. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Liang, L.-Y.; Yan, P.-F.; Li, G.-M.; Wang, C.; Ma, D.-Y. Study on ligation of copper complexes of the quinolone antibacterial drugs and octamolybdates POMs. Polyhedron 2012, 31, 422–430. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Chen, Z.-F.; Shi, S.-M.; Luo, H.-S.; Zhong, D.-C.; Zou, H.-L.; Liang, H. Synthesis, crystal structure of polyoxovanadate complex of ciprofloxacin: V4O10(μ2-O)2[VO(H-Ciprof)2]2 · 13H2O by hydrothermal reaction. Inorg. Chem. Commun. 2007, 10, 1269–1272. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Tu, F.; Chen, J.; Li, Y. Study of the first antibacterial agent pipemidic acid modifying Keggin polyoxometalate. Inorg. Chem. Commun. 2011, 14, 1192–1195. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Liang, L.-Y.; Li, X.; Zhang, Yu.; Yan, H.; Chen, G. Ligation of the quinolone antibacterial agent pipemidic acid to Keggin polyoxotungstates. Polyhedron 2011, 30, 1657–1662. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Li, X.; Zhou, Y.-H.; Yan, P.-F.; Li, G.-M.; Wang, C. The introduction of antibacterial drug pipemidic acid into the POM field: Syntheses, characterization and antitumor activity. Solid State Sci. 2011, 13, 1972–1977. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chen, Z.-F.; Xiong, R.-G.; Xue, Z.; Ju, H.-X.; You, X.-Z. A mononuclear complex of norfloxacin with silver(I) and its properties. Inorg. Chem. Commun. 2003, 6, 819–822. [Google Scholar] [CrossRef]
- Refat, M.S. Synthesis and characterization of norfloxacin-transition metal complexes (group 11, IB): Spectroscopic, thermal, kinetic and biological activity. Spectrochim. Acta Part A 2007, 5, 1393–1405. [Google Scholar]
- Badea, M.; Olar, R.; Marinescu, D.; Uivarosi, V.; Iacob, D. thermal decomposition of some biologically active complexes of ruthenium (III) with quinolone derivatives. J. Therm. Anal. Calorim. 2009, 97, 735–739. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Marinescu, D.; Uivarosi, V.; Nicolescu, T.O.; Iacob, D. Thermal study of some new quinolone ruthenium(III) complexes with potential cytostatic activity. J. Therm. Anal. Calorim. 2010, 99, 829–834. [Google Scholar] [CrossRef]
- Chen, Z.F.; Xiong, R.G.; Zuo, J.; Guo, Z.; You, X.; Fun, H.K. X-ray crystal structures of Mg2+ and Ca2+ dimers of the antibacterial drug norfloxacin. J. Chem. Soc. Dalton Trans. 2000, 22, 4013–4014. [Google Scholar]
- Chen, Z.F.; Zhou, H.L.; Liang, H.; Li, Y.; Xiong, R.G.; You, X.Z. Crystallographic report: Bis(norfloxacin)dilead(II) tetranitrate, [Pb2(H-Norf)2(ONO2)4]. Appl. Organomet. Chem. 2003, 17, 883–884. [Google Scholar] [CrossRef]
- Qu, Z.-R.; Zhao, H.; Xing, L.-X.; Wang, X.-S.; Chen, Z.-F.; Yu, Z.; Xiong, R.-G.; You, X.-Z. Two polymeric complexes of norfloxacin with iron(II) and their magnetic properties. Eur. J. Inorg. Chem. 2003, 16, 2920–2923. [Google Scholar]
- Chen, Z.-F.; Yu, L.-C.; Zhong, D.-C.; Liang, H.; Zhu, X.-H.; Zhu, Z.-Y. An unprecedented 1D ladder-like silver (I) coordination polymer with ciprofloxacin. Inorg. Chem. Commun. 2006, 9, 839–843. [Google Scholar] [CrossRef]
- Gerasimenko, A.V.; Polishchuk, A.V.; Volkova, L.M.; Karaseva, E.T.; Karasev, V.E. Synthesis and structure of nalidixium tetrachloroantimonate monohydrate, (C12H13N2O3)SbCl4· H2O. Russ. J. Coord. Chem. 2008, 34, 8–13. [Google Scholar] [CrossRef]
- Gerasimenko, A.V.; Polishchuk, A.V.; Karaseva, E.T.; Karasev, V.E. Crystal Structure and Spectroscopic Properties of Ciprofloxacinium Pentachloroantimonate(III) Monohydrate (C17H19N3O3F)SbCl5· H2O. Russ. J. Coord. Chem. 2008, 34, 647–652. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Crystal structure and characterization of the bismuth (III) compound with quinolone family member (ciprofloxacin). Antibacterial study. J. Inorg. Biochem. 1997, 66, 241–245. [Google Scholar] [CrossRef]
- Turel, I.; Golić, L.; Bukovec, P.; Gubina, M. antibacterial tests of bismuth(III)-quinolone (ciprofoxacin, cf ) compounds against Helicobacter pylori and some other bacteria. Crystal structure of (cfH2)2[Bi2Cl10]·4H2O. J. Inorg. Biochem. 1998, 71, 53–60. [Google Scholar] [CrossRef]
- Turel, I.; Guber, K.; Leban, I.; Bukovec, N. Synthesis, crystal structure, and characterization of tree novel compounds of quinolone family member (norfloxacin). J. Inorg. Biochem. 1996, 61, 197–212. [Google Scholar] [CrossRef]
- Vasiliev, D.; Golovnev, N.N. Synthesis and Structure of C17H22FN3O32+CuCl42-. J. Struct. Chem. 2010, 51, 177–180. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Klinchar, G.; Bukovec, N.; Zalar, S. Synthesis, crystal structure and characterization of two metal-quinolone compound. J. Inorg. Biochem. 1997, 66, 77–82. [Google Scholar] [CrossRef]
- Zupančič, M.; Turel, I.; Bukovec, P.; White, A.J.P.; Williams, D.J. Synthesis and characterization of 2 novel zinc (II) complexes with ciprofloxacin. Crystal-structure of [C17H19N3O3F]2·[ZnCl4]·2H2O. Croat. Chem. Acta 2001, 74, 61–74. [Google Scholar]
- Polishchuk, A.V.; Karaseva, E.T.; Cherednichenko, A.I.; Gerasimenko, A.V.; Karasev, V.E. Crystal structure and X-ray photoelectron spectroscopy of ciprofloxacinium tetrachloroaurate monohydrate. Russ. J. Coord. Chem. 2011, 37, 215–222. [Google Scholar] [CrossRef]
- Olivera, M.E.; Mazzieri, M.R.; Manzo, R.H. New pharmaceutical fluoroquinolone derivatives hydrochloride of aluminum complexes of ciprofloxacin and norfloxacin. STP Pharma Sci. 2000, 10, 251–256. [Google Scholar]
- Olivera, M.E.; Allemandi, D.A.; Manzo, R.H. Intrinsic dissolution rate and intestinal permeability of metallic complexes of norfloxacin and ciprofloxacin in relation to their formulation. Acta Farm. Bonaerense 2000, 19, 185–191. [Google Scholar]
- Alovero, F.L.; Olivera, M.E.; Manzo, R.H. In vitro pharmacodynamic properties of a fluoroquinolone pharmaceutical derivative: Hydrochloride of ciprofloxacin–aluminium complex. Int. J. Antimicrob. Agents 2003, 21, 446–451. [Google Scholar] [CrossRef]
- Höffken, G.; Borner, K.; Glatzel, P.D.; Koeppe, P.; Lode, H. Reduced enteral absorption of ciprofloxacin in the presence of antacids (letter). Eur. J. Clin. Microbiol. 1985, 4, 345. [Google Scholar]
- Kara, M.; Hassinoff, B.B.; Mckay, D.N.; Campbell, N.R.C. Clinical and chemical interactions between iron preparations and ciprofloxacin. Brit. J. Clin. Pharmacol. 1991, 31, 257–261. [Google Scholar] [CrossRef]
- Wallis, S.C.; Charles, B.G.; Gahan, L.R.; Filippich, L.J.; Bredhauer, M.G.; Duckworth, P.A. Interaction of norfloxacin with divalent and trivalent pharmaceutical cations, In vitro complexation and in vivo pharmacokinetic studies in the dog. J. Pharm. Sci. 1996, 85, 803–809. [Google Scholar] [CrossRef]
- Davies, M.; Maesen, F.P.V. Drug interactions with quinolones. Rev. Infect. Dis. 1989, 2, S1083–S1090. [Google Scholar] [CrossRef]
- Nix, D.E.; Watson, W.A.; Handy, L.; Frost, R.W.; Rescott, D.L.; Goldstein, H.R. The effect of sucralfate pretreatment on the pharmacokinetics of ciprofloxacin. Pharmacotherapy 1989, 9, 377–380. [Google Scholar]
- Polk, R.E.; Healey, D.P.; Sahai, J.; Drwal, L.; Racht, E. Effect of ferrous sulphate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob. Agents Chemother. 1989, 33, 1841–1844. [Google Scholar] [CrossRef]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. III. Complexation of lomefloxacin with various metal ions and the effect of metal ion complexation on aqueous solubility. Int. J. Pharmaceut. 1992, 87, 203–213. [Google Scholar] [CrossRef]
- Ross, D.; Elkinton, S.; Knaub, S.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. VI. Effect of metal-ion complexation on octanol-1-ol-water partitioning. Int. J. Pharmaceut. 1993, 93, 131–138. [Google Scholar] [CrossRef]
- akelj, S.; Berginc, K.; Uršič, D.; Veber, M.; Kristl, A. Metal cation-fluoroquinolone complexes do not permeate through the intestinal absorption barrier. J. Pharm. Biomed. Anal. 2010, 53, 655–659. [Google Scholar] [CrossRef]
- Pallù, G.; Valisena, S.; Ciarrocchi, G.; Gatto, B.; Palumbo, M. Quinolone binding to DNA is mediated by magnesium ions. Proc. Natl. Acad. Sci. USA 1992, 89, 9671–9675. [Google Scholar] [CrossRef]
- Skauge, T.; Turel, I.; Sletten, E. Interaction between ciprofloxacin and DNA mediated by Mg2+-ions. Inorg. Chim. Acta 2002, 339, 239–247. [Google Scholar] [CrossRef]
- Song, G.; Yan, Q.; He, Y. Studies on interaction of norfloxacin, Cu2+ and DNA by spectral methods. J. Fluoresc. 2005, 15, 673–678. [Google Scholar] [CrossRef]
- Drevenšek, P.; Turel, I.; Poklar Ulrih, N. Influence o copper (II) and magnesium (II) ions on the ciprofloxacin binding to DNA. J. Inorg. Biochem. 2003, 96, 407–415. [Google Scholar] [CrossRef]
- Guo, D.-S.; Jing, B.Y.; Yuan, X.-Y. Influence of Mg2+ and Cu2+ on the interaction between quinolone and calf thymus DNA. J. Fluoresc. 2011, 21, 113–118. [Google Scholar] [CrossRef]
- Song, G.; He, Y.; Cai, Z. The interaction between levofloxacine hydrochloride and DNA mediated by Cu2+. J. Fluoresc. 2004, 14, 705–710. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Qin, J.; Lu, L.-L. Influence of metal ions on the interaction between gatifloxacin and calf thymus DNA. Spectrochim. Acta A 2010, 75, 520–524. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Guo, D.-S.; Wang, L.L. Influence of Mg2+ and Cd2+ on the interaction between sparfloxacin and calf thymus DNA. Spectrochim. Acta A 2008, 69, 1130–1135. [Google Scholar] [CrossRef]
- Guo, D.-S.; Yuan, X.-Y.; Wu, J.-B. Influence of Cr(III) and Cr(VI) on the interaction between sparfloxacin and calf thymus DNA. J. Inorg. Biochem. 2007, 101, 644–648. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, X.; Liu, Q.; Wang, G. Interaction between pazufloxacin and DNA mediated by copper(II) ions. J. Fluoresc. 2008, 18, 701–706. [Google Scholar] [CrossRef]
- Sissi, C.; Andreolli, M.; Cecchetti, V.; Fravolini, A.; Gatto, B.; Palumbo, M. Mg2+-mediated binding of 6-Substituted quinolones to DNA: Relevance to biological activity. Bioorg. Med. Chem. 1998, 6, 1555–1561. [Google Scholar] [CrossRef]
- Robles, J.; Martin-Polo, J.; Avarez-Valtierra, L.; Hinojosa, L.; Mendoza-Diaz, G. A theoretical-experimental study on the structure and activity of certain quinolones and the interaction of their Cu(II)-complexes on a DNA model. Met. Based Drugs 2000, 7, 301–311. [Google Scholar] [CrossRef]
- Sissi, C.; Marangon, E.; Chemello, A.; Noble, C.G.; Maxwell, A.; Palumbo, M. The effects of metal ions on the structure and stability of the DNA gyrase B protein. J. Mol. Biol. 2005, 353, 1152–1160. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 2009, 37, 702–711. [Google Scholar] [CrossRef]
- Wohlkonig, A.; Chan, P.F.; Fosberry, A.P.; Homes, P.; Huang, J.; Kranz, M.; Leydon, V.R.; Miles, T.J.; Pearson, N.D.; Perera, R.L.; et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 2010, 17, 1152–1153. [Google Scholar] [CrossRef]
- Aldred, K.J.; McPherson, S.A.; Turnbough, C.L., Jr.; Kerns, R.J.; Osheroff, N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: Mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013, 41, 4628–4639. [Google Scholar] [CrossRef]
- Lecomte, S.; Baron, M.H.; Chenon, M.T.; Compry, C.; Moreau, N.J. Effect of magnesium complexation by fluoroquinolones on their antibacterial properties. Antimicrob. Agents Chemother. 1994, 38, 2810–2816. [Google Scholar] [CrossRef]
- Alkaysi, H.N.; Abdel-Hay, M.H.; Sheikh Salem, M.; Gharaibeh, A.M.; Na’was, T.E. Chemical and biological investigations of metal ion interaction with norfloxacin. Int. J. Pharmaceut. 1992, 87, 73–77. [Google Scholar] [CrossRef]
- Tumer, M.; Koksal, H.; Sener, M.K.; Serin, S. Antimicrobial activity studies of the binuclear metal complexes derived from tridentate schiff base ligands. Transit. Met. Chem. 1999, 24, 414–420. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, J.; Iqbal, S.; Ijaz, N. In vitro antibacterial studies of ciprofloxacin-imines and their complexes with Cu(II), Ni(II), Co(II), and Zn(II). Turk. J. Biol. 2007, 31, 67–72. [Google Scholar]
- Patel, N.H.; Parekh, H.M.; Patel, M.N. Synthesis, physicochemical characteristics, and biocidal activity of some transition metal mixed-ligand complexes with bidentate (NO and NN) Schiff bases. Pharm. Chem. J. 2007, 41, 78–82. [Google Scholar] [CrossRef]
- Takiff, H.; Guerrero, E. Current prospects for the fluoroquinolones as first-line tuberculosis therapy. Antimicrob. Agents. Chemother. 2011, 55, 5421–5429. [Google Scholar] [CrossRef]
- Chang, K.C.; Yew, W.W.; Chan, R.C. Rapid assays for fluoroquinolone resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2010, 65, 1551–1561. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir. Med. 2009, 103, 1777–1790. [Google Scholar] [CrossRef]
- Saha, D.K.; Padhye, S.; Anson, C.E.; Powell, A.K. Hydrothermal synthesis, crystal structure, spectroscopy, electrochemistry and antimycobacterial evaluation of the copper (II) ciprofloxacin complex: [Cu(cf)2(BF4)2]·6H2O. Inorg. Chem. Commun. 2002, 5, 1022–1027. [Google Scholar] [CrossRef]
- Sulochana, S.; Rahman, F.; Paramasivan, C.N. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J. Chemother. 2005, 17, 169–173. [Google Scholar]
- Nishizawa, T.; Suzuki, H.; Hibi, T. Quinolone-based third-line therapy for Helicobacter pylori eradication. J. Clin. Biochem. Nutr. 2009, 44, 119–124. [Google Scholar] [CrossRef]
- Berning, M.; Krasz, S.; Miehlke, S. Should quinolones come first in Helicobacter pylori therapy? Ther. Adv. Gastroenterol. 2011, 4, 103–114. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Ergül, B.; Koçak, E.; Taş, A.; Filik, L.; Köklü, S. Bismuth, moxifloxacin, tetracycline, lansoprazole quadruple first line therapy for eradication of H. pylori: A prospective study. Clin. Res. Hepatol. Gastroenterol. 2013. [Google Scholar] [CrossRef]
- Turel, I.; Kljun, J.; Perdih, F.; Morozova, E.; Bakulev, V.; Kasyanenco, N.; Byl, J.A.W.; Osheroff, N. First ruthenium organometallic complex of antibacterial agent ofloxacin. Crystal structure and interactions with DNA. Inorg. Chem. 2010, 49, 10750–10752. [Google Scholar] [CrossRef]
- Herold, C.; Ocker, M.; Ganslmayer, M.; Gerauer, H.; Hahn, E.G.; Schuppan, D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 2002, 86, 443–448. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. The quinolone family: From antibacterial to anticancer agents. Curr. Med. Chem. Anticancer Agents. 2003, 3, 439–450. [Google Scholar] [CrossRef]
- Thadepalli, H.; Salem, F.; Chuah, S.K.; Gollapudi, S. Antitumor activity of trovafloxacin in an animal model. In Vivo 2005, 19, 269–276. [Google Scholar]
- Ahmed, A.; Daneshtalab, M. Nonclassical Biological Activities of Quinolone Derivatives. J. Pharm. Pharmaceut. Sci. 2012, 15, 52–72. [Google Scholar]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327357. [Google Scholar]
- Kljun, J.; Bytzek, A.K.; Kandioller, W.; Bartel, C.; Jakupec, M.A.; Hartinger, C.G.; Keppler, B.K.; Turel, I. Physicochemical studies and anticancer potency of ruthenium η-p-cymene complexes containing antibacterial quinolones. Organometallics 2011, 30, 2506–2512. [Google Scholar] [CrossRef]
- Eboka, C.J.; Aigbavboa, S.O.; Akerele, J.O. Colorimetric determination of the fluoroquinolones. J. Antimicrob. Chemother. 1997, 39, 639–641. [Google Scholar] [CrossRef]
- Fratini, L.; Schapoval, E.E.S. Ciprofloxacin determination by visible light spectrophotometry using iron(III) nitrate. Int. J. Pharmaceut. 1996, 127, 279–282. [Google Scholar] [CrossRef]
- Sultan, S.M.; Suliman, F.-E.O. Chemometric optimization and flow injection method for the determination of norfloxacin in drug formulations. Analyst 1993, 118, 573–576. [Google Scholar] [CrossRef]
- Al-Momani, I.F.; Haj-Hussein, A.T.; Tahtamouni, A.N. Flow injection spectrophotometric and chromatographic determination of ciprofloxacin and norfloxacin in pharmaceutical formulations. J. Flow Inject. Anal. 2008, 25, 151–155. [Google Scholar]
- García, M.S.; Albero, M.I.; Sánchez-Pedreño, C.; Abuherba, M.S. Flow injection spectrophotometric determination of ofloxacin in pharmaceuticals and urine. Eur. J. Pharm. Biopharm. 2005, 61, 87–93. [Google Scholar] [CrossRef]
- Sultan, S.M.; Suliman, F.-E.O. Flow injection spectrophotometric determination of the antibiotic ciprofloxacin in drug formulations. Analyst 1992, 117, 1523–1526. [Google Scholar] [CrossRef]
- Suliman, F.E.O.; Sultan, S.M. Sequential injection technique employed for stoichiometric studies, optimization and quantitative determination of some fluoroquinolones antibiotics complexed with iron (III) in sulfuric acid media. Talanta 1996, 43, 559–568. [Google Scholar] [CrossRef]
- El Khateeb, S.Z.; Razek, S.A.; Amer, M.M. Stability-indicating methods for the spectrophotometric determination of norfloxacin. J. Pharm. Biomed. Anal. 1998, 17, 829–840. [Google Scholar] [CrossRef]
- Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.; Sheribah, Z.A. Derivative spectrophotometric analysis of 4-quinolone antibacterials in formulations and spiked biological fluids by their Cu (II) complexes. J. AOAC Int. 2001, 84, 368–375. [Google Scholar]
- El Walily, A.F.M.; Belal, S.F.; Bakry, R.S. Spectrophotometric and spectrofluorimetric estimation of ciprofloxacin and norfloxacin by ternary complex formation with eosin and palladium(II). J. Pharm. Biomed. Anal. 1996, 14, 561–569. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakao, M.; Nakahara, R.; Nishioka, Y.; Ikeda, C.; Fujita, Y. Spectrophotometric determination of quinolone antibiotics by an association complex formation with aluminum(III) and erythrosine. Anal. Sci. 2009, 25, 125–128. [Google Scholar] [CrossRef]
- Uivarosi, V.; Monciu, C.M. The gravimetric and spectrophotometric assay of ofloxacin using ammonium reineckate. Rev. Chim. 2005, 56, 726–730. [Google Scholar]
- Uivarosi, V.; Monciu, C.M. Studies on the gravimetric and spectrophotometric analysis of norfloxacin using ammonium reineckate. Rev. Chim. 2009, 60, 132–136. [Google Scholar]
- Salem, H. Spectrofluorimetric, atomic absorption spectrometric and spectrophotometric determination of some fluoroquinolones. Am. J. Appl. Sci. 2005, 2, 719–729. [Google Scholar] [CrossRef]
- Cordoba-Diaz, M.; Cordoba-Borrego, M.; Cordoba-Diaz, D. modification of fluorescent properties of norfloxacin in the presence of certain antacids. J. Pharm. Biomed. Anal. 1998, 18, 565–571. [Google Scholar] [CrossRef]
- Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.; el-Enany, N. Spectrofluorimetric analysis of certain 4-quinolone in pharmaceuticals and biological fluids. Pharm. Acta Helv. 2000, 74, 371–377. [Google Scholar] [CrossRef]
- Djurdjević, P.T.; Jelikić-Stankov, M.; Stankov, D. Fluorescence reaction and complexation equilibria between norfloxacin and aluminium (III) ion in chloride medium. Anal. Chim. Acta 1995, 300, 253–259. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Carpena, J. Determination of norfloxacin in real samples by different pectrofluorimetric techniques. Analyst 1997, 122, 705–708. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Yang, J.; Sun, S. The fluorescence characteristic of the yttrium–norfloxacin system and its analytical application. J. Pharm. Biomed. Anal. 2005, 38, 528–531. [Google Scholar] [CrossRef]
- Tong, C.; Zhuo, X.; Liu, W.; Wu, J. Synchronous fluorescence measurement of enrofloxacin in the pharmaceutical formulation and its residue in milks based on the yttrium (III)-perturbed luminescence. Talanta 2010, 82, 1858–1863. [Google Scholar] [CrossRef]
- Beltyukova, S.; Teslyuk, O.; Egorova, A.; Tselik, E. Solid-phase luminescence determination of ciprofloxacin and norfloxacin in biological fluids. J. Fluoresc. 2002, 12, 269–271. [Google Scholar] [CrossRef]
- Tong, C.; Xiang, G. Sensitive determination of enoxacin by its enhancement effect on the fluorescence of terbium(III)–sodium dodecylbenzene sulfonate and its luminescence mechanism. J. Luminesc. 2007, 126, 575–580. [Google Scholar] [CrossRef]
- Kaur, K.; Singh Saini, S.; Malik, A.K.; Singh, B. Micelle enhanced and terbium sensitized spectrofluorimetric determination of danofloxacin in milk using molecularly imprinted solid phase extraction. Spectrochim. Acta A 2012, 96, 790–795. [Google Scholar] [CrossRef]
- Zhao, H.C.; Ding, F.; Wang, X.; Ju, H.; Li, A.; Jin, L.P. A study on silver nanoparticles-sensitized fluorescence and second-order scattering of the complexes of Tb(III) with ciprofloxacin and its applications. Spectrochim. Acta Part A 2008, 70, 332–336. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, H.; Jin, L.; Zheng, D. Study of the influence of silver nanoparticles on the second-order scattering and the fluorescence of the complexes of Tb(III) with quinolones and determination of the quinolones. Anal. Chim. Acta 2006, 566, 136–143. [Google Scholar] [CrossRef]
- Attia, M.S.; Essawy, A.A.; Youssef, A.O. Europium-sensitized and simultaneous pH-assisted spectrofluorimetric assessment of ciprofloxacin, norfloxacin and gatifloxacin in pharmaceutical and serum samples. J. Photochem. Photobiol. A 2012, 236, 26–34. [Google Scholar] [CrossRef]
- Dong, P.; Na, X.; Fu, B.; Wang, L. Rapid europium-sensitized fluorescent determination of ulifloxacin, the active metabolite of prulifloxacin, in human serum and urine. J. Pharm. Anal. 2011, 1, 46–50. [Google Scholar]
- Attia, M.S.; Youssef, A.O.; Essawy, Amr A.; Abdel-Mottaleb, M.S.A. A highly luminescent complexes of Eu(III) and Tb(III) with norfloxacin and gatifloxacin doped in sol–gel matrix: A comparable approach of using silica doped Tb(III) and Eu(III) as optical sensor. J. Luminesc. 2012, 132, 2741–2746. [Google Scholar] [CrossRef]
- Jelikić-Stankov, M.; Stankov, D.; Djurdjević, P. Determination of pefloxacin in serum by time-resolved fluorimetry. Pharmazie 1999, 54, 73–74. [Google Scholar]
- Luiz, F.C.L.; Garcia, L.S.; Goes Filho, L.S.; Teixeira, L.R.; Louro, S.R.W. Fluorescence studies of gold(III)-norfloxacin complexes in aqueous solutions. J. Fluoresc. 2011, 21, 1933–1940. [Google Scholar] [CrossRef]
- Pan, B.; Han, X.; Wu, M.; Peng, H.; Zhang, D.; Li, H.; Xing, B. Temperature dependence of ofloxacin fluorescence quenching and complexation by Cu(II). Environ. Pollut. 2012, 171, 168–173. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Jiang, Y.C. Flow injection flame atomic spectrometry for the indirect analysis of norfloxacin. Atom. Spectroscop. 2001, 22, 429–432. [Google Scholar]
- Al-Ghannam, S.M. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of some fluoroquinolone antibacterials using ammonium reineckate. Spectrochim. Acta A 2008, 69, 1188–1194. [Google Scholar] [CrossRef]
- Issopoulos, B.P. spectrophotometric determination of norfloxacin in pharmaceutical formulations. Analyst 1989, 114, 627–630. [Google Scholar] [CrossRef]
- Wang, N.-X.; Wang, L.; Jiang, W.; Ren, Y.-Z.; Si, Z.-K.; Qiu, X.-X.; Du, G.-Y.; Qi, P. Determination of neodymium, holmium and erbium in mixed rare earths by norfloxacin. Fresenius J. Anal. Chem. 1998, 361, 821–824. [Google Scholar] [CrossRef]
- Guo, C.; Lang, A.; Wang, L.; Jiang, W. The co-luminescence effect of a europium (III)–lanthanum (III)–gatifloxacin–sodium dodecylbenzene sulfonate system and its application for the determination of trace amount of europium(III). J. Luminesc. 2010, 130, 591–597. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Chen, Y. Detection of mercury ions (Hg2+) in urine using a terbium chelate fluorescent probe. Sens. Actuators B 2011, 156, 120–125. [Google Scholar] [CrossRef]
- Beltyukova, S.V.; Egorova, A.V.; Teslyuk, O.I. Europium(III) and terbium(III) chelates of quinolonecarboxylic acid derivatives as labels for immunofluorimetric assay. J. Anal. Chem. 2000, 55, 682–685. [Google Scholar] [CrossRef]
- Tong, C.; Hu, Z.; Liu, W. Enoxacin–Tb3+ complex as an environmentally friendly fluorescence probe for DNA and its application. Talanta 2007, 71, 816–821. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 2013, 18, 11153-11197. https://doi.org/10.3390/molecules180911153
Uivarosi V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules. 2013; 18(9):11153-11197. https://doi.org/10.3390/molecules180911153
Chicago/Turabian StyleUivarosi, Valentina. 2013. "Metal Complexes of Quinolone Antibiotics and Their Applications: An Update" Molecules 18, no. 9: 11153-11197. https://doi.org/10.3390/molecules180911153




