Anti-Peroxyl Radical Quality and Antibacterial Properties of Rooibos Infusions and Their Pure Glycosylated Polyphenolic Constituents
Abstract
:1. Introduction

2. Results and Discussion
2.1. Antioxidant Performance of Pure Polyphenol Compounds and Rooibos Infusions
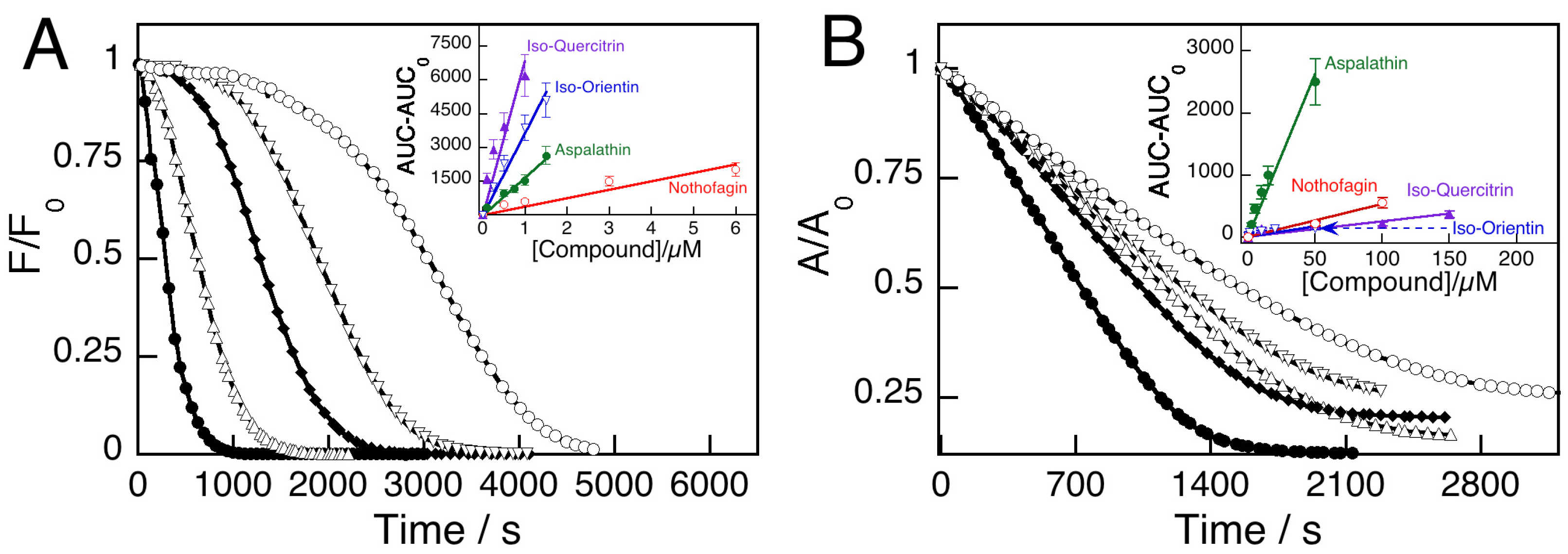
| Compound | Classification | ORAC-FL | ORAC-PGR | ORAC-PGR/ORAC-FL |
|---|---|---|---|---|
| Aspalathin | Dihydrochalcone | 2.65 ± 0.13 (0.5) α | 0.18 ± 0.015 (5.0) ß | 0.068 |
| Nothofagin | 0.47 ± 0.040 (1.0) | 0.020 ± 0.002 (≈25) | 0.042 | |
| Orientin | Flavone | 0.86 ± 0.070 (0.7) | 0.0038 ± 0.0003 (≈150) | 0.0044 |
| Vitexin | 4.57 ± 0.065 (0.3) | 0.0078 ± 0.001 (≈55) | 0.0017 | |
| Iso-orientin | 4.83 ± 0.30 (0.3) | 0.0077 ± 0.001 (50) | 0.0015 | |
| Isoquercitrin | Flavonol | 8.22 ± 0.10 (0.1) | 0.0074 ± 0.0005 (50) | 0.0010 |
| Rutin | 6.01 ± 0.25 * | 0.009 | 0.0015 | |
| Hyperoside | 5.50 ± 0.4 (0.1) | <0.0015 (>50) | 0.0003 | |
| Quercetin | 8.90 † (0.07) | 1.0 † (0.5) | 0.11 |
- Do Asp and Not control the antioxidant activity of natural rooibos infusions
- Is there a correlation between the ORAC ratios of the natural infusions with those of the artificial infusions prepared with the pure compounds in the same concentrations as present in the natural infusion
| Compound | Green Rooibos | Fermented Rooibos | ||
|---|---|---|---|---|
| g/100 g dried extract | mg/mL * | g/100 g dried extract | mg/mL * | |
| Aspalathin | 10.019 | 0.1067 | 0.383 | 0.0031 |
| Nothofagin | 1.731 | 0.0184 | 0.151 | 0.0012 |
| Orientin | 0.86 | 0.0092 | 1.206 | 0.0097 |
| Vitexin | 0.173 | 0.0018 | 0.217 | 0.0017 |
| Iso-orientin | 1.068 | 0.0114 | 1.205 | 0.0097 |
| Rutin | 0.404 | 0.0043 | 0.064 | 0.0005 |
| Hyperoside | 0.217 | 0.0023 | 0.13 | 0.0010 |
| Infusions | Phenolic content † | ORAC-FL †† | ORAC-PGR †† | ORAC-PGR/ORAC-FL |
|---|---|---|---|---|
| Green rooibos | 280 ± 20 | 1840 ± 168 | 176 ± 4.0 | 0.096 |
| Fermented rooibos | 249 ± 15 | 1520 ± 60 | 68 ± 5.0 | 0.044 |
| Artificial green rooibos | 156.9 * | 600 ± 100 (0.28) α | 88 ± 12 (1.4) ß | 0.14 |
| Artificial fermented rooibos | 27.3 * | 160 ± 36 (0.045) | 52 ± 4.0 (0.22) | 0.33 |

- The ORAC-FL indices of the artificial infusions were much smaller than the respective indices measured for the natural rooibos infusions;
- The ORAC-PGR indices of the artificial infusion were less than the respective indices of the natural rooibos infusions, but the differences were less pronounced than in the case of ORAC-FL.
2.2. Antimicrobial Activity of Pure Compounds and Rooibos Infusions
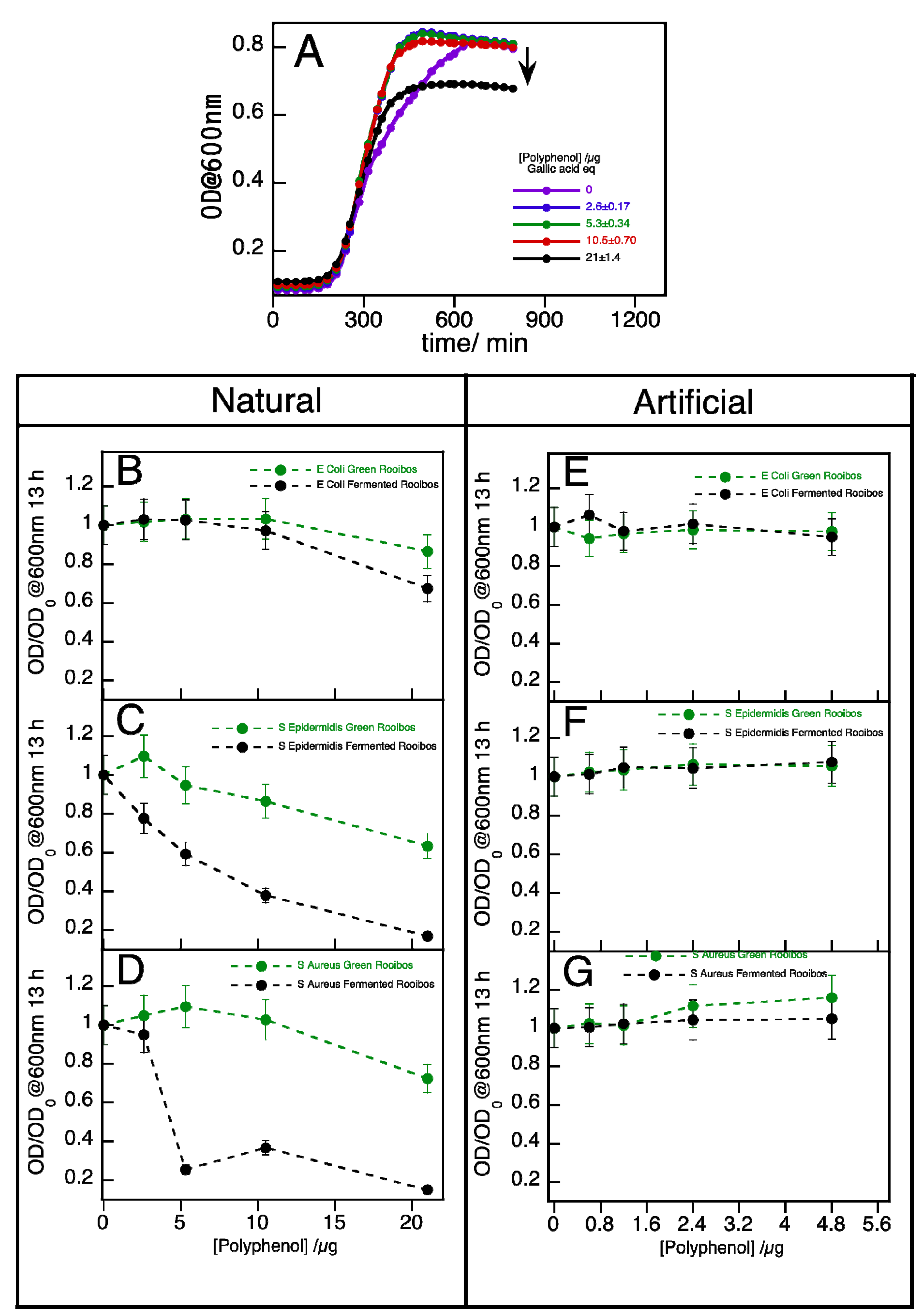
3. Experimental
3.1. Instruments, Chemicals and Infusions
3.2. Polyphenols
3.3. Infusions Preparation
3.4. Artificial Infusion Preparation
3.5. Total Phenol Content and Soluble Solids
3.6. Polyphenolic Content Determination
3.7. ORAC assays


- AUC = area under the curve in the presence of a tested compound or infusion;
- AUC0 = area under the curve of control experiment (without additives);
- AUCGallic acid = area under the curve in the presence of gallic acid
- [Gallic acid] = concentration of gallic acid expressed as mol/L,
- [Compound] = concentration of tested compound expressed as mol/L, and
- f = is the dilution factor, equal to the ratio between the total volume of the reaction sample and the added infusion volume.
3.8. Antimicrobial Activity of Rooibos Extracts and Pure Compounds
4. Conclusions
Abbreviations
| ORAC | oxygen radical absorbance capacity |
| PGR | pyrogallol red |
| FL | fluorescein |
| AUC | area under the curve |
| ORAC-PGR | oxygen radical absorbance capacity based on pyrogallol red consumption |
| ORAC-FL | oxygen radical absorbance capacity based on fluorescein consumption |
| ORAC ratio | ORAC-PGR/ORAC-FL |
| AAPH | 2,2'-azo-bis(2-amidinopropane) dihydrochloride |
| Asp | aspalathin |
| Not | nothofagin |
| Ori | orientin |
| Vit | vitexin |
| I-Or | iso-orientin |
| Hyp | hyperoside |
| Rut | rutin |
| I-Qc | isoquercitrin |
| Qc | quercetin |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| Trolox | 6-hydroxy-2,5,8-tetramethylchroman-2-carboxylic acid |
Acknowledgments
Conflicts of Interest
References
- Prior, R.L.; Cao, G. Antioxidant capacity and polyphenolic components of teas: Implications for altering in vivo antioxidant status. Proc. Soc. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbo, R.; Jimenez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Warren, C.P.W. Antioxidant effects of herbs. The Lancet 1999, 353, 676. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chong, K.L.; Tan, J.B.L.; Wong, S.K. Antioxidant properties of tropical and temperate herbal teas. J. Food Compos. Anal. 2010, 23, 185–189. [Google Scholar] [CrossRef]
- Speisky, H.; Rocco, C.; Carrasco, C.; Lissi, E.A.; López-Alarcón, C. Antioxidant screening of medicinal herbal teas. Phytother. Res. 2006, 20, 462–467. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E.A. Evaluation of the antioxidant capacity of herbal teas by a procedure based on the bleaching of ABTS radical cations. J. Chil. Chem. Soc. 1995, 40, 375–381. [Google Scholar]
- Roginsky, V.; Barsukova, T. Chain-breaking antioxidant capability of some beverages as determined by the Clark electrode technique. J. Med. Food 2001, 4, 219–229. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Lissi, E. A novel and simple ORAC methodology based on the interaction of pyrogallol red with peroxyl radicals. Free Rad. Res. 2006, 40, 979–985. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Lissi, E. Interaction of pyrogallol red with peroxyl radicals. A basis for a simple methodology for the evaluation of antioxidant capabilities. Free Rad. Res. 2005, 39, 729–736. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Ortiz, R.; Benavides, J.; Mura, E.; Lissi, E. Use of the ORAC-pyrogallol red/ORAC-fluorescein ratio to assess the quality of antioxidants in Chilean wines. J. Chil. Chem. Soc. 2011, 56, 764–767. [Google Scholar] [CrossRef]
- Omata, Y.; Saito, Y.; Yoshida, Y.; Niki, E. Simple assessment of radical scavenging capacity of beverages. J. Agric. Food Chem. 2008, 56, 3386–3390. [Google Scholar] [CrossRef]
- Liu, Z.Q. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef]
- Perez, D.D.; Leighton, F.; Aspee, A.; Aliaga, C.; Lissi, E. A comparison of methods employed to evaluate antioxidant capabilities. Biol. Res. 2000, 33, 71–77. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Hermsdorff, H.H.M.; Puchau, B.; Volp, A.C.P.; Barbosa, K.B.F.; Bressan, J.; Zulet, M.A.; Martinez, J.A. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab. 2011, 8, 1–8. [Google Scholar] [CrossRef]
- Poblete, A.; Lopez-Alarcon, C.; Lissi, E.; Campos, A.M. Oxygen radical antioxidant capacity (ORAC) values of herbal teas obtained employing different methodologies can provide complementary data. J. Chil. Chem. Soc. 2009, 54, 154–157. [Google Scholar]
- Alarcon, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; Lopez-Alarcon, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Jimenez, L.; Alarcon, E.; Trevithick-Sutton, C.; Gandhi, N.; Scaiano, J.C. Effect of γ-radiation on green onion DNA integrity: Role of ascorbic acid and polyphenols against nucleic acid damage. Food Chem. 2011, 128, 735–741. [Google Scholar] [CrossRef]
- Cheney, R.H.; Scholtz, E. Rooibos tea, a South African contribution to world beverages. Econ. Bot. 1963, 17, 186–194. [Google Scholar] [CrossRef]
- Morton, J.F. Rooibos tea, Aspalathus linearis, a caffeineless, low-tannin beverage. Econ. Bot. 1983, 37, 164–173. [Google Scholar] [CrossRef]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.-C.; Ding, Y.; Green, I.R.; Gelderblom, W.C. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Joubert, E.; Beelders, T.; de Beer, D.; Malherbe, C.J.; de Villiers, A.J.; Sigge, G.O. Variation in phenolic content and antioxidant activity of fermented rooibos herbal tea infusions: Role of production season and quality grade. J. Agric. Food Chem. 2012, 60, 9171–9179. [Google Scholar] [CrossRef]
- Krafczyk, N.; Woyand, F.; Glomb, M.A. Structure–antioxidant relationship of flavonoids from fermented rooibos. Mol. Nutr. Food Res. 2009, 53, 635–642. [Google Scholar] [CrossRef]
- Joubert, E.; Gelderblom, W.C.; Louw, A.; de Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef]
- Standley, L.; Winterton, P.; Marnewick, J.L.; Gelderblom, W.C.; Joubert, E.; Britz, T.J. Influence of processing stages on antimutagenic and antioxidant potentials of rooibos tea. J. Agric. Food Chem. 2001, 49, 114–117. [Google Scholar] [CrossRef]
- Joubert, E. HPLC quantification of the dihydrochalcones, aspalathin and nothofagin in rooibos tea (Aspalathus linearis) as affected by processing. Food Chem. 1996, 55, 403–411. [Google Scholar] [CrossRef]
- Joubert, E.; Winterton, P.; Britz, T.J.; Gelderblom, W.C. Antioxidant and pro-oxidant activities of aqueous extracts and crude polyphenolic fractions of rooibos (Aspalathus linearis). J. Agric. Food Chem. 2005, 53, 10260–10267. [Google Scholar] [CrossRef]
- Joubert, E.; Winterton, P.; Britz, T.J.; Ferreira, D. Superoxide anion and α-α-diphenyl-β-picrylhydrazyl radical scavenging capacity of rooibos (Aspalathus linearis) aqueous extracts, crude phenolic fractions, tannin and flavonoids. Food Res. Int. 2004, 37, 133–138. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, Antioxidants, Redox reactions, And methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D. Phenolic content and antioxidant acitivity of rooibos food ingredient extracts. J. Food Compos. Anal. 2012, 27, 45–51. [Google Scholar] [CrossRef]
- Scheepers, S. Anti-Microbial Activity of Rooibos tea (Aspalathus linearis) on Food Spoilage Organisms and Potenial Pathogens; University of Stellenbosch: Stellenbosch, South Africa, 2001. [Google Scholar]
- Eagle, H. The binding of penicillin in relation to its cytotoxic action. III. The binding of penicillin by mammalian cells in tissue culture (HeLa and L strains). J. Exp. Med. 1954, 100, 117–124. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, H.; Osawa, T. Dihydrochalcones: Evaluation as novel radical scavenging antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.R.M.M.; van der Vijgh, W.J.F.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Kozlowski, D.; Trouillas, P.; Calliste, C.; Marsal, P.; Lazzaroni, R.; Duroux, J.-L. Density functional theory study of the conformational, Electronic, And antioxidant properties of natural chalcones. J. Phys. Chem. A 2007, 111, 1138–1145. [Google Scholar] [CrossRef]
- Pratt, D. Natural antioxidants of soybean and other oilseeds. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Plenum Press: New York, NY, USA, 1980; pp. 283–292. [Google Scholar]
- Blauz, A.; Pilaszek, T.; Grzelak, A.; Dragan, A.; Bartosz, G. Interaction between antioxidants in assays of total antioxidant capacity. Food Chem. Toxicol. 2008, 46, 2365–2368. [Google Scholar] [CrossRef]
- Yanishlieva-Maslarova, N.V. Inhibiting oxidation. In Antioxidants in Food—Practical Applications; Pokorny, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing: Cornwall, UK, 2001; pp. 22–47. [Google Scholar]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Coetzee, G.; Marx, I.J.; Pengilly, M.; Bushula, V.S.; Joubert, E.; Bloom, M. Effect of rooibos and honeybush tea extracts against Botrytis cinerea. S. Afr. J. Enol. Vitic. 2008, 29, 33–38. [Google Scholar]
- Ankolekar, C.; Johnson, D.; Pinto Mda, S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef]
- Yepremyan, A.; Salehani, B.; Minehan, T.G. Concise total syntheses of aspalathin and nothofagin. Org. Lett. 2010, 12, 1580–1583. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; Gonzalez-Bejar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef]
- Wikler, M.A. Performance standards for antimicrobial susceptibility testing: Fifteenth informational supplement, Approved Standard—Ninth EditionClinical and Laboratory Standards Institute: Wayne, PA, USA, 2005; Volume 25. [Google Scholar]
- Sample Availability: Samples of the polyphenols are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simpson, M.J.; Hjelmqvist, D.; López-Alarcón, C.; Karamehmedovic, N.; Minehan, T.G.; Yepremyan, A.; Salehani, B.; Lissi, E.; Joubert, E.; Udekwu, K.I.; et al. Anti-Peroxyl Radical Quality and Antibacterial Properties of Rooibos Infusions and Their Pure Glycosylated Polyphenolic Constituents. Molecules 2013, 18, 11264-11280. https://doi.org/10.3390/molecules180911264
Simpson MJ, Hjelmqvist D, López-Alarcón C, Karamehmedovic N, Minehan TG, Yepremyan A, Salehani B, Lissi E, Joubert E, Udekwu KI, et al. Anti-Peroxyl Radical Quality and Antibacterial Properties of Rooibos Infusions and Their Pure Glycosylated Polyphenolic Constituents. Molecules. 2013; 18(9):11264-11280. https://doi.org/10.3390/molecules180911264
Chicago/Turabian StyleSimpson, Madeline J., Daisy Hjelmqvist, Camilo López-Alarcón, Nadja Karamehmedovic, Thomas G. Minehan, Akop Yepremyan, Baback Salehani, Eduardo Lissi, Elizabeth Joubert, Klas I. Udekwu, and et al. 2013. "Anti-Peroxyl Radical Quality and Antibacterial Properties of Rooibos Infusions and Their Pure Glycosylated Polyphenolic Constituents" Molecules 18, no. 9: 11264-11280. https://doi.org/10.3390/molecules180911264





