A New Lignan Glucoside from the Whole Plants of Salvia Scapiformis
Abstract
:1. Introduction
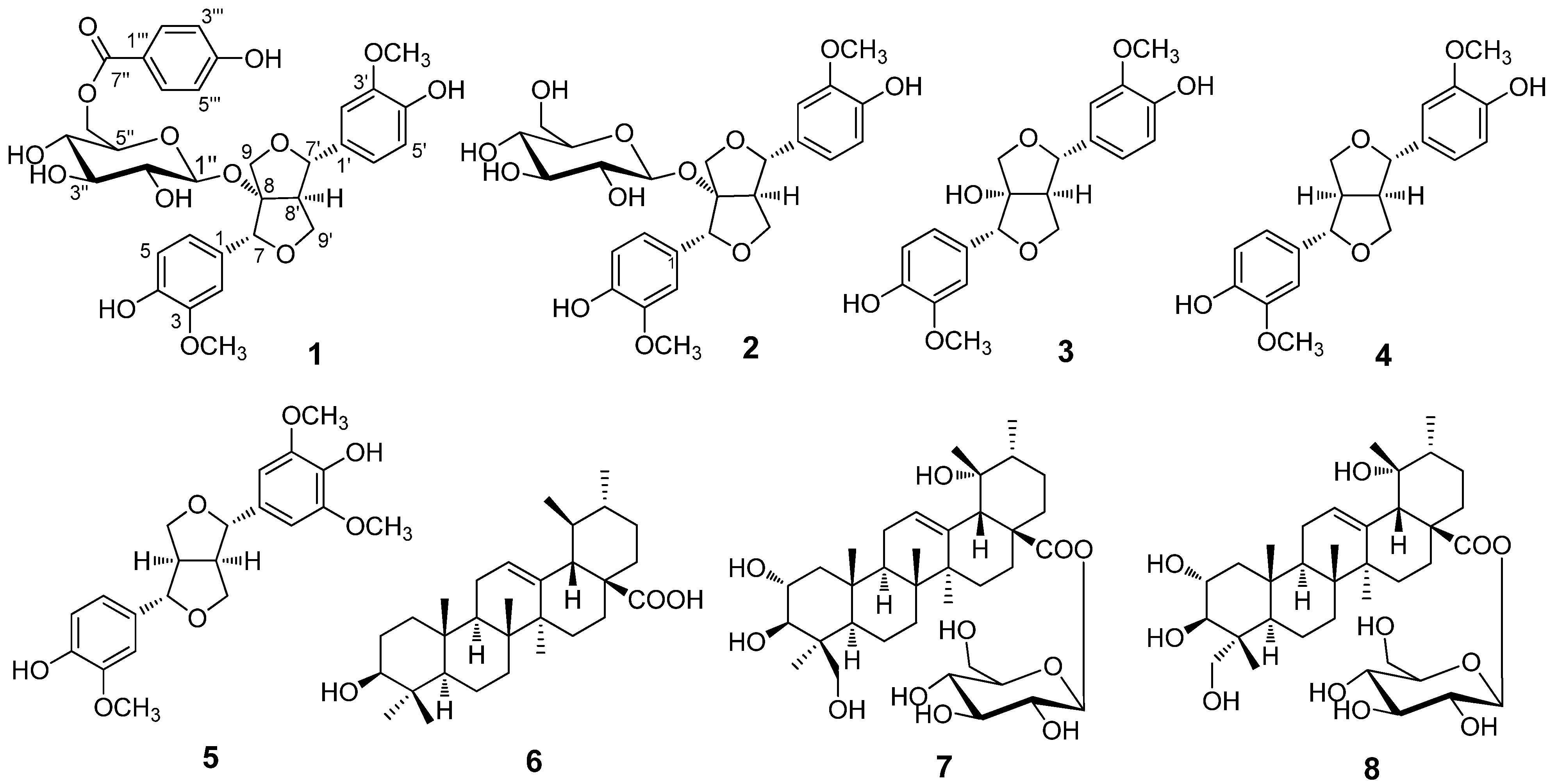
2. Results and Discussion
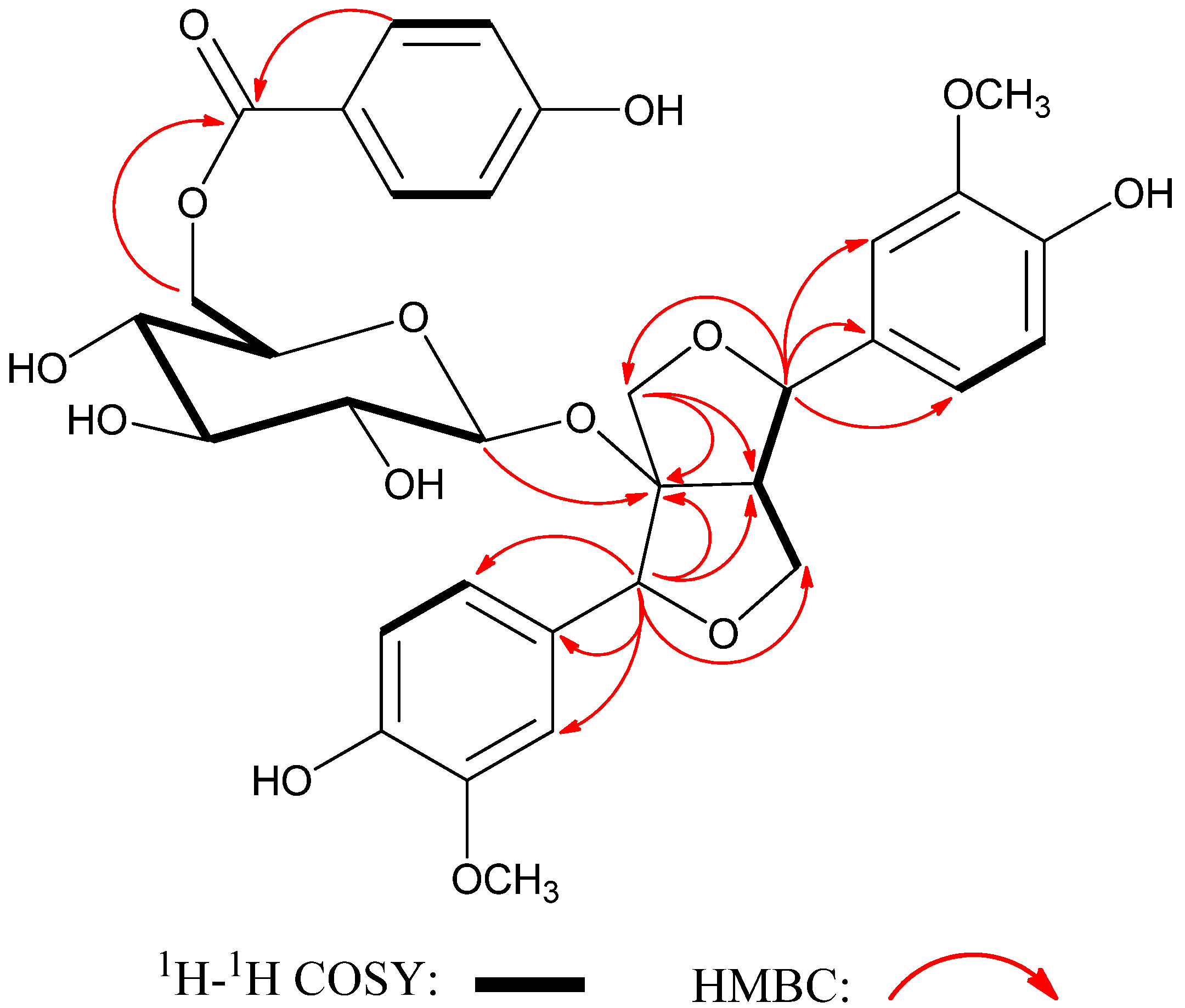

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
 +4.35 (c 0.16, CH3OH); UV (CH3OH) λmax (lg ε) 207 (4.82), 234 (4.26), 259 (4.21) nm; IR (film) vmax 3359, 2943, 1700, 1607, 1517, 1453,1369, 1277 cm−1; 1H and 13C-NMR data, see Table 1; positive HRESIMS m/z 679.1985 [M + Na]+ (calcd for C33H36O14Na, 679.2003).
+4.35 (c 0.16, CH3OH); UV (CH3OH) λmax (lg ε) 207 (4.82), 234 (4.26), 259 (4.21) nm; IR (film) vmax 3359, 2943, 1700, 1607, 1517, 1453,1369, 1277 cm−1; 1H and 13C-NMR data, see Table 1; positive HRESIMS m/z 679.1985 [M + Na]+ (calcd for C33H36O14Na, 679.2003).| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC | |
|---|---|---|---|---|---|---|
| 1 | 128.9, s | 8′α | 3.40, ddd (8.0, 6.0, 5.1) | 60.4, d | ||
| 2 | 7.03, d (1.7) | 113.6, d | 9′α | 4.46, dd (9.2, 8.0) | 72.4, t | |
| 3 | 149.4, s | 9′β | 3.76, dd (9.2, 6.0) | |||
| 4 | 147.5, s | -OCH3 | 3.84, s | 56.7, q | ||
| 5 | 6.72, d (8.1) | 115.5, d | 3.82, s | 56.6, q | ||
| 6 | 6.83 (overlapped) | 122.1, d | G1′′ | 4.38, d (7.7) | 100.2, d | |
| 7β | 4.66, s | 89.5, d | G2′′ | 3.04, m | 74.9, d | |
| 8 | 99.3, s | G3′′ | 3.16, m | 78.3, d | ||
| 9α | 4.38, d (10.4) | 73.6, t | G4′′ | 3.16, m | 72.0, d | |
| 9β | 3.85, d (10.4) | G5′′ | 3.23, dd (15.2, 7.6) | 75.7, d | ||
| 1′ | 133.2, s | G6′′a | 4.52, dd (11.8,1.8) | 65.1, t | ||
| 2′ | 7.01, d (1.7) | 111.0, d | G6′′b | 4.20, dd (11.8, 7.5) | ||
| 3′ | 148.5, s | 1′′′ | 122.2, s | |||
| 4′ | 147.4, s | 2′′′, 6′′′ | 7.88, d (8.8) | 133.0, d | ||
| 5′ | 6.77, d (8.1) | 116.3, d | 3′′′, 5′′′ | 6.83, d (8.8) | 116.4, d | |
| 6′ | 6.83 (overlapped) | 120.2, d | 4′′′ | 163.8, s | ||
| 7′β | 4.77, d (5.1) | 87.2, d | 7′′′ | 167.9, s |
3.4. Hydrolysis of Compound 1 and Determination of the Absolute Configuration of the Sugar Moiety
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Li, X.W.; Ian, C.H. Lamiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; Volume 17, pp. 50–299. [Google Scholar]
- Fang, Z.X.; Liao, C.L. Medicinal Flora of Enshi; Hubei Science and Technology Press: Wuhan, China, 2006; Volume 2, pp. 292–293. [Google Scholar]
- Choi, S.U.; Yang, M.C.; Lee, K.H.; Kim, K.H.; Lee, K.R. Lignan and terpene constituents from the aerial parts of Saussurea pulchella. Arch. Pharm. Res. 2007, 30, 1067–1074. [Google Scholar] [CrossRef]
- Wang, H.B.; Mayer, R.; Rücker, G.; Neugebauer, M. Bisepoxylignan glycosides from Stauntonia hexaphylla. Phytochemistry 1993, 34, 1621–1624. [Google Scholar] [CrossRef]
- Cowan, S.; Stewart, M.; Abbiw, D.K.; Latif, Z.; Sarker, S.D.; Nash, R.J. Lignans from Strophanthus gratus. Fitoterapia 2001, 72, 80–82. [Google Scholar] [CrossRef]
- Khan, K.A.; Shoeb, A. A lignan from Lonicera hypoleuca. Phytochemistry 1985, 24, 628–630. [Google Scholar] [CrossRef]
- Jang, D.S.; Kim, J.M.; Lee, G.Y.; Kim, J.; Kim, J.S. Ursane-type triterpenoids from the aerial parts of Potentilla discolor. Agric. Chem. Biotechnol. 2006, 49, 48–50. [Google Scholar]
- Zhou, X.H.; Kasai, R.; Ohtani, K.; Tanaka, O.; Nie, R.L.; Yang, C.H.; Zhou, J.; Yamasaki, K. Oleanane and ursane glucosides from Rubus species. Phytochemistry 1992, 31, 3642–3644. [Google Scholar] [CrossRef]
- Reher, G.; Buděšínský, M. Triterpenoids from plants of the Sanguisorbeae. Phytochemistry 1992, 31, 3909–3914. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhu, H.L.; Zhang, S.W.; Zhang, X.H.; Yu, Q.; Xuan, L.J. Abietane diterpenoids from Clerodendrum bungei. J. Nat. Prod. 2008, 71, 755–759. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Kiem, P.V.; Minh, C.V.; Kim, N.; Park, S.; Lee, H.Y.; Kim, E.S.; Kim, Y.H.; Kim, S.; Koh, Y. Diarylheptanoids and flavonoids from Viscum album inhibit LPS-Stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. J. Nat. Prod. 2013, 76, 495–502. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lai, Y.; Ding, S.; Qian, H.; Zhang, J.; Xue, Y.; Luo, Z.; Yao, G.; Zhang, Y. A New Lignan Glucoside from the Whole Plants of Salvia Scapiformis. Molecules 2013, 18, 11377-11383. https://doi.org/10.3390/molecules180911377
Lai Y, Ding S, Qian H, Zhang J, Xue Y, Luo Z, Yao G, Zhang Y. A New Lignan Glucoside from the Whole Plants of Salvia Scapiformis. Molecules. 2013; 18(9):11377-11383. https://doi.org/10.3390/molecules180911377
Chicago/Turabian StyleLai, Yongji, Shuiping Ding, Huiqin Qian, Jinwen Zhang, Yongbo Xue, Zengwei Luo, Guangmin Yao, and Yonghui Zhang. 2013. "A New Lignan Glucoside from the Whole Plants of Salvia Scapiformis" Molecules 18, no. 9: 11377-11383. https://doi.org/10.3390/molecules180911377





