Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Analysis of Extracts
| Extracts | Phenolic compounds (mg/kg) | |||
|---|---|---|---|---|
| Chlorogenic acid | Caffeic acid | p-Coumaric acid | Ferulic acid | |
| Tomatoes | ND | 6,765.7 ± 4 a | 10,209.8 ± 3 a | 4,786.7 ± 3 a |
| Sauce | 101.5 ± 2 a | ND | 7.9 ± 1 b | ND |
| Ketchup | 181.3 ± 2 b | 55.6 ± 2 b | 8.9 ± 1 b | 7.9 ± 1 b |
| Juice | 233.8 ± 3 c | 55.2 ± 1 b | 25.1 ± 2 c | 25.9 ± 2 c |
| Pomace | 75.6 ± 1 a | 51.2 ± 2 b | 24.9 ± 2 c | 2.3 ± 1 b |
2.2. Antiplatelet Activity of Bioactive Compounds
| Compounds | Platelet antiaggregant activity (%) | |||
|---|---|---|---|---|
| ADP | Collagen | TRAP-6 | AA | |
| Chlorogenic acid | 69 ± 5 * | 50 ± 5 * | 19 ± 4 * | 22 ± 5 * |
| Caffeic acid | 35 ± 5 * | 42 ± 6 * | 25 ± 5 * | 20 ± 3 * |
| Ferulic acid | 47 ± 4 * | 36 ± 3 * | NS | NS |
| p-Coumaric acid | 71 ± 6 * | 69 ± 5 * | 41 ± 6 * | NS |
2.3. Antiplatelet Activity of Tomatoes and Its Derivates
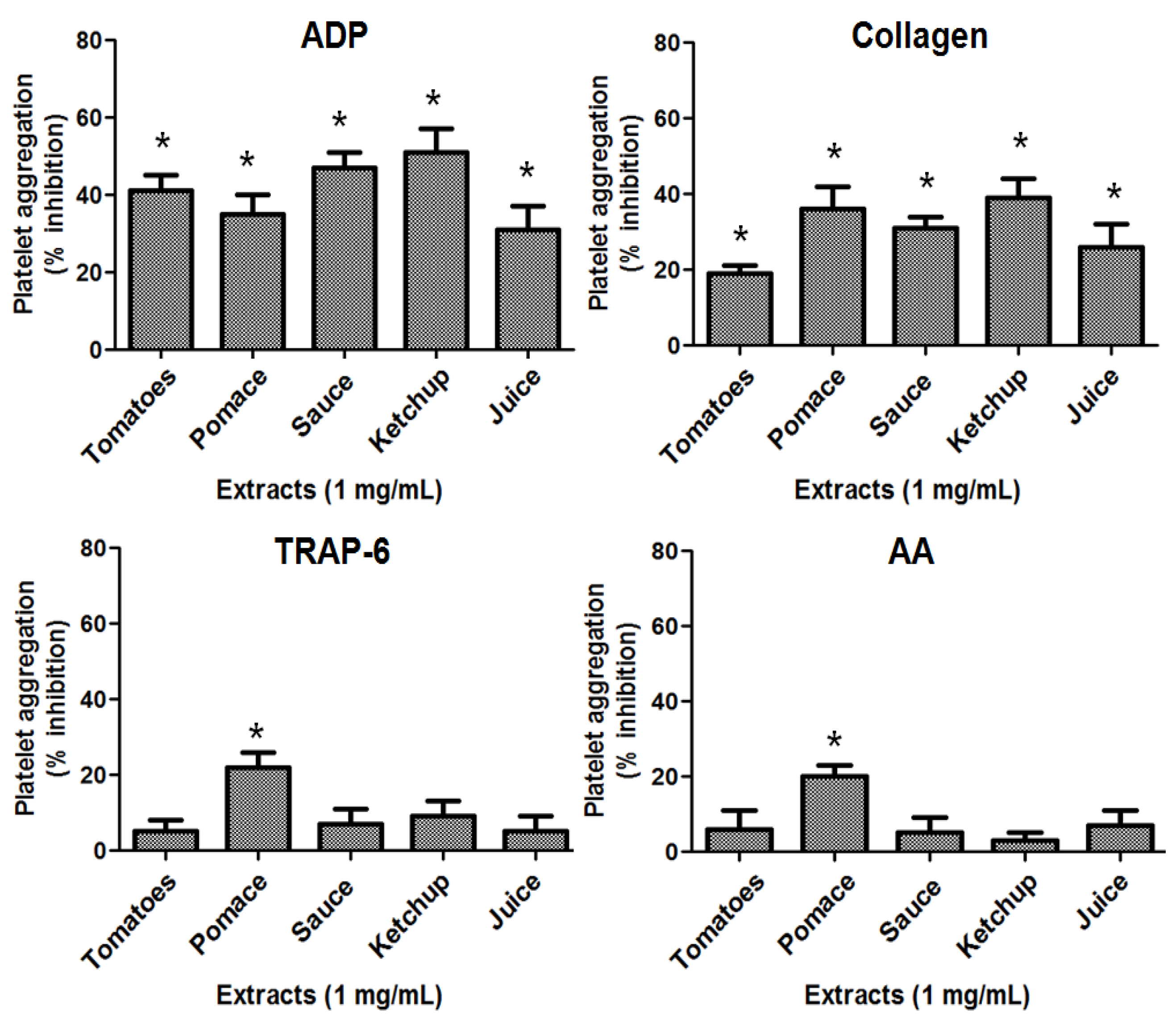
2.4. Antithrombotic Activity of Pomace Extract
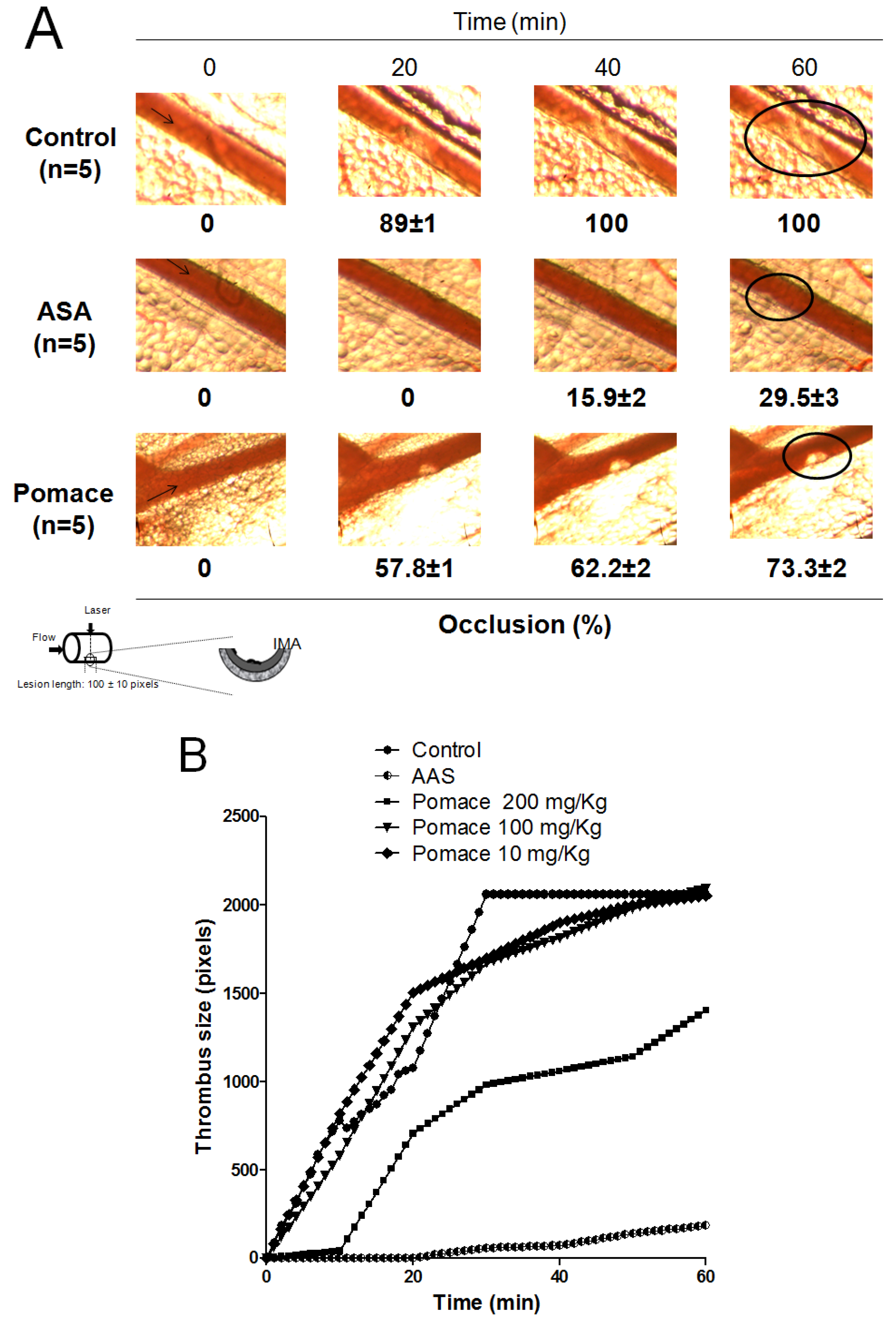
3. Experimental
3.1. Chemicals and Reagents
3.2. Processing Material
3.3. Preparation of Extracts
3.4. Instrument
3.5. Preparation of Human Platelet Suspensions
3.6. Platelet Aggregation Assay
3.7. In Vivo Murine Model of Thrombosis
3.8. Statistical Analysis
4. Conclusion
Acknowledgments
Conflicts of Interest
References
- World Health Organization, WHO publishes definitive atlas on global heart disease and stroke epidemic. In Indian J. Med. Sci.; 2004; 58, pp. 405–406.
- Laslett, L.; Alagona, P.; Clark, B.; Drozda, J.; Saldivar, F.; Wilson, S.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Andrés, V.; Pello, O.; de Mora, J.; Palomo, I. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2013, 24, 255–262. [Google Scholar] [CrossRef]
- Willoughby, S.; Holmes, A.; Loscalzo, J. Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2002, 1, 273–288. [Google Scholar] [CrossRef]
- Nishijima, K.; Kiryu, J.; Tsujikawa, A.; Miyamoto, K.; Honjo, M.; Tanihara, H.; Nonaka, A.; Yamashiro, K.; Katsuta, H.; Miyahara, S.; et al. Platelets adhering to the vascular wall mediate postischemic leukocyte-endothelial cell interactions in retinal microcirculation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 977–984. [Google Scholar] [CrossRef]
- Jackson, S.P.; Nesbitt, W.S.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 2009, 7, 17–20. [Google Scholar] [CrossRef]
- Zoungas, S.; McGrath, B.P.; Branley, P.; Kerr, P.G.; Muske, C.; Wolfe, R.; Atkins, R.C.; Nicholls, K.; Fraenkel, M.; Hutchison, B.G.; et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: A multicenter, randomized, controlled trial. J. Am. Coll. Cardiol. 2006, 47, 1108–1116. [Google Scholar] [CrossRef]
- Hartley, L.; Igbinedion, E.; Holmes, J.; Flowers, N.; Thorogood, M.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2013, 6, CD009874. [Google Scholar]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Fuentes, E.; Astudillo, L.A.; Gutierrez, M.I.; Contreras, S.O.; Bustamante, L.O.; Rubio, P.I.; Moore-Carrasco, R.; Alarcon, M.A.; Fuentes, J.A.; Gonzalez, D.E.; et al. Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood Coagul. Fibrinolysis 2012, 23, 109–117. [Google Scholar] [CrossRef]
- Fuentes, E.; Castro, R.; Astudillo, L.; Carrasco, G.; Alarcon, M.; Gutierrez, M.; Palomo, I. Bioassay-guided isolation and HPLC determination of bioactive compound that relate to the antiplatelet activity (adhesion, secretion, and aggregation) from solanum lycopersicum. Evid.-Based Complement. Altern. Med. 2012, 2012, 147031. [Google Scholar]
- Fuentes, E.; Alarcon, M.; Astudillo, L.; Valenzuela, C.; Gutierrez, M.; Palomo, I. Protective mechanisms of guanosine from solanum lycopersicum on agonist-induced platelet activation: Role of sCD40L. Molecules 2013, 18, 8120–8135. [Google Scholar] [CrossRef]
- Le Gall, G.; Colquhoun, I.J.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E. Metabolite profiling of tomato (Lycopersicon esculentum) using 1H NMR spectroscopy as a tool to detect potential unintended effects following a genetic modification. J. Agric. Food Chem. 2003, 51, 2447–2456. [Google Scholar] [CrossRef]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (solanum lycopersicum) and pomace from industrial tomato processing. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar]
- Lavelli, V.; Torresani, M.C. Modelling the stability of lycopene-rich by-products of tomato processing. Food Chem. 2011, 125, 529–535. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.; Flessa, S.; Gillespie, D.M.; Jewell, W.T.; Huebner, B.; Bertow, D.; Ebeler, S.E. Processing effects on lycopene content and antioxidant activity of tomatoes. J. Agric. Food Chem. 2001, 49, 3713–3717. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Collins, B.; Hollidge, C. Antithrombotic drug market. Nat. Rev. Drug Discov. 2003, 2, 11–12. [Google Scholar] [CrossRef]
- Palomo, I.; Toro, C.; Alarcon, M. The role of platelets in the pathophysiology of atherosclerosis (Review). Mol. Med. Rep. 2008, 1, 179–184. [Google Scholar]
- Hubbard, G.P.; Wolffram, S.; Lovegrove, J.A.; Gibbins, J.M. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J. Thromb. Haemost. 2004, 2, 2138–2145. [Google Scholar] [CrossRef]
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: An overview. Am. J. Clin. Nutr. 2003, 78, 544S–551S. [Google Scholar]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; de Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds-recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Akl, E.A.; Crowther, M.; Gutterman, D.D.; Schuunemann, H.J. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012, 141, 7S–47S. [Google Scholar] [CrossRef]
- Born, G.V.; Cross, M.J. The aggregation of blood platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar]
- Przyklenk, K.; Whittaker, P. Adaptation of a photochemical method to initiate recurrent platelet-mediated thrombosis in small animals. Lasers Med. Sci. 2007, 22, 42–45. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (chlorogenic acid, caffeic acid, ferulic acid and p-coumaric acid) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricán, A.; Santos, L.S.; Alarcón, M.; Palomo, I. Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity. Molecules 2013, 18, 11526-11536. https://doi.org/10.3390/molecules180911526
Fuentes E, Forero-Doria O, Carrasco G, Maricán A, Santos LS, Alarcón M, Palomo I. Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity. Molecules. 2013; 18(9):11526-11536. https://doi.org/10.3390/molecules180911526
Chicago/Turabian StyleFuentes, Eduardo, Oscar Forero-Doria, Gilda Carrasco, Adolfo Maricán, Leonardo S. Santos, Marcelo Alarcón, and Iván Palomo. 2013. "Effect of Tomato Industrial Processing on Phenolic Profile and Antiplatelet Activity" Molecules 18, no. 9: 11526-11536. https://doi.org/10.3390/molecules180911526






