Combined Effects of Green Tea Extracts, Green Tea Polyphenols or Epigallocatechin Gallate with Acarbose on Inhibition against α-Amylase and α-Glucosidase in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Each Component Alone against α-Glucosidase and α-Amylase
| Components | IC50 (μg/mL) | |
|---|---|---|
| Single | Combined | |
| GTE | 4.421 ± 0.018 * | 2.104 ± 0.007 * |
| GTP | 10.019 ± 0.017 * | 3.999 ± 0.006 * |
| EGCG | 5.272 ± 0.009 * | 2.083 ± 0.004 * |
| Acarbose | 4,822.783 ± 26.042 | 2,291.587 ± 13.014 *† (GTE) |
| 1,925.614 ± 9.875 *† (GTP) | ||
| 1,906.454 ± 6.892 *† (EGCG) | ||
| Components | IC50 (μg/mL) | |
|---|---|---|
| Single | Combined | |
| GTE | 4,020.157 ± 172.363 | 2,845.987 ± 154.07 |
| GTP | 1,370.812 ± 59.081 * | 609.463 ± 20.351 * |
| EGCG | 1,849.612 ± 73.475 * | 1,094.802 ± 55.992 * |
| 1,974.612 ± 18.653 *† (GTE) | ||
| Acarbose | 2,715.654 ± 24.709 | 1,206.974 ± 11.395 *† (GTP) |
| 1,614.753 ± 14.691 *† (EGCG) | ||
2.2. Combined Effects of Each Sample and Acarbose against α-Amylase and α-Glucosidase
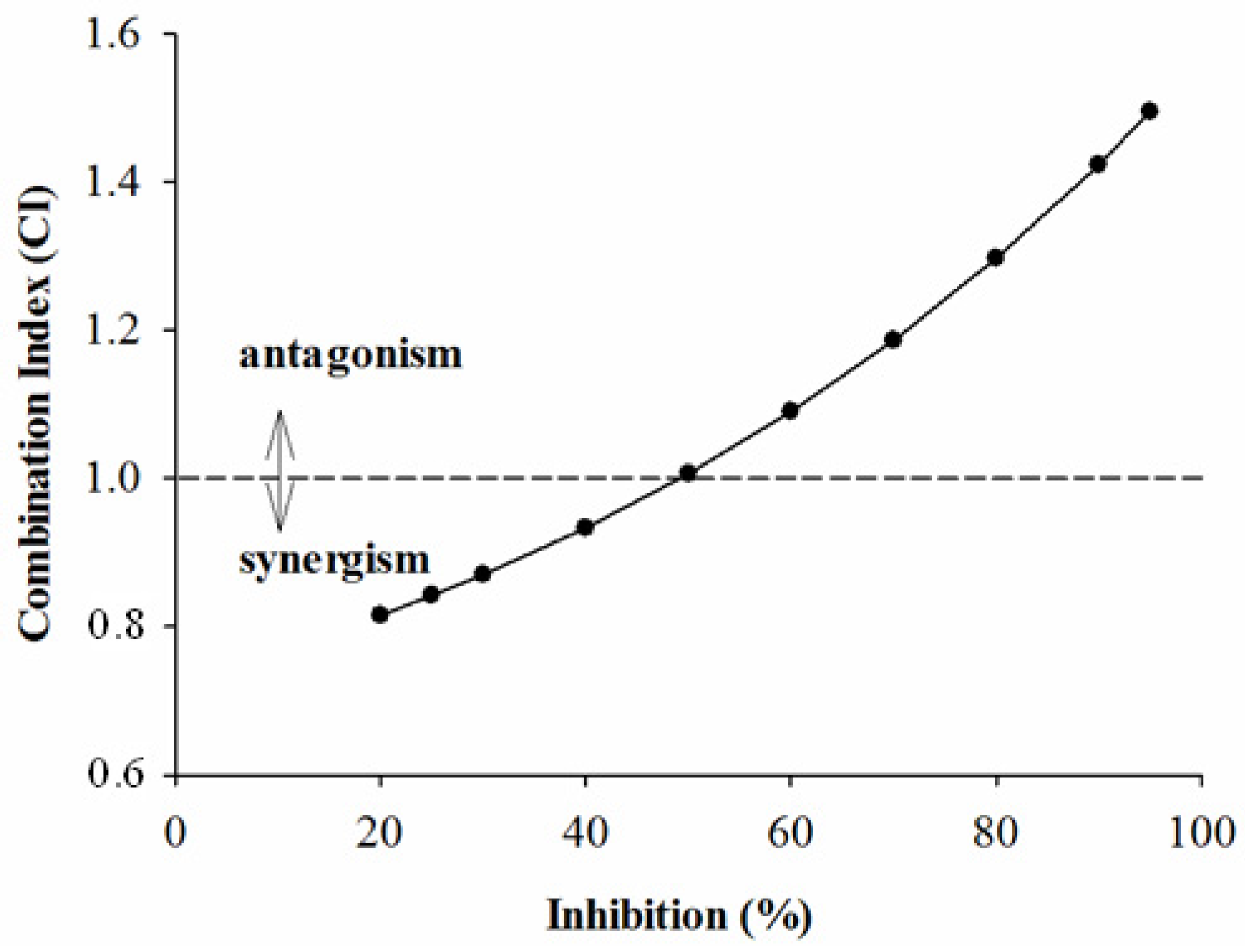
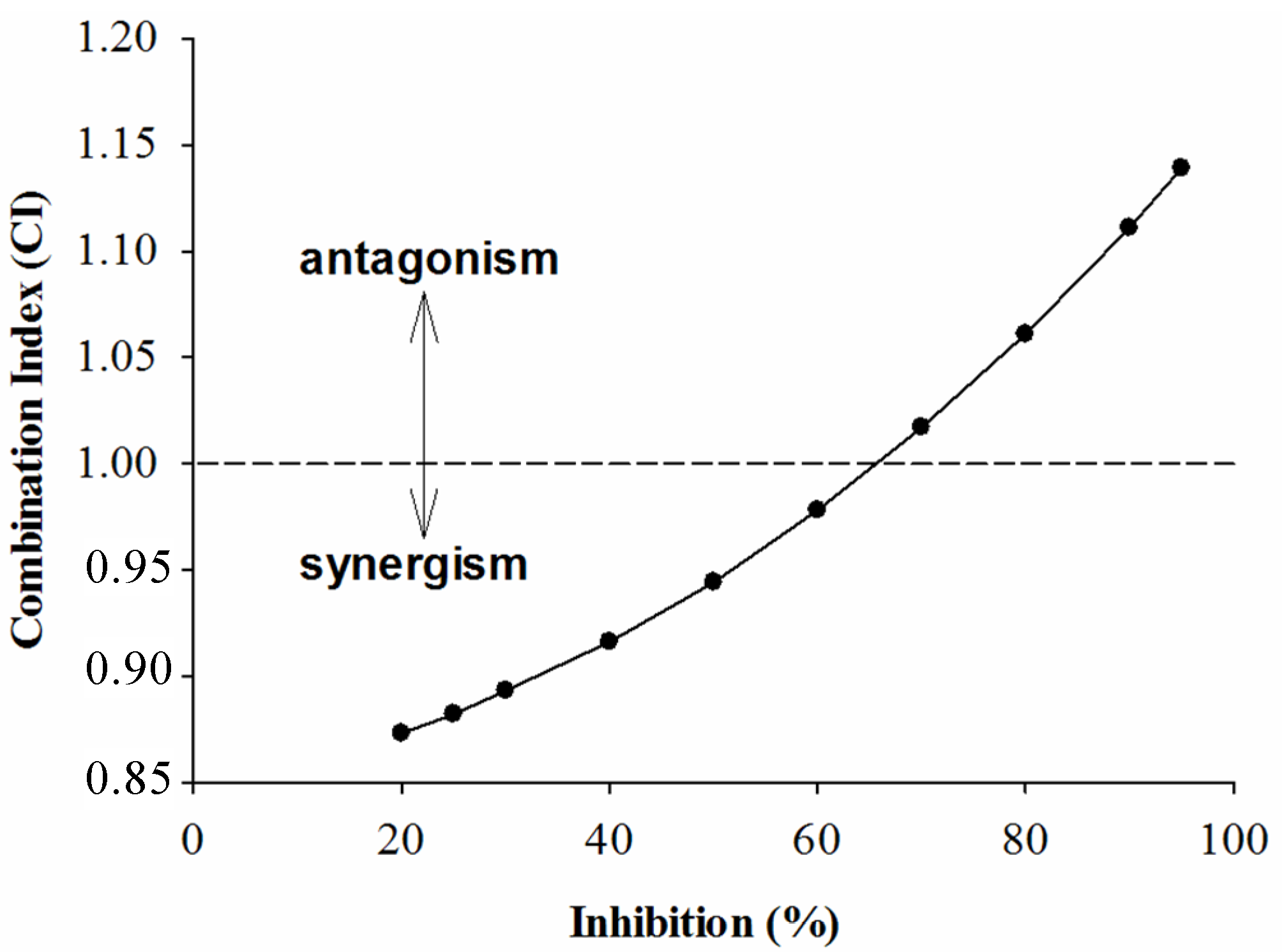
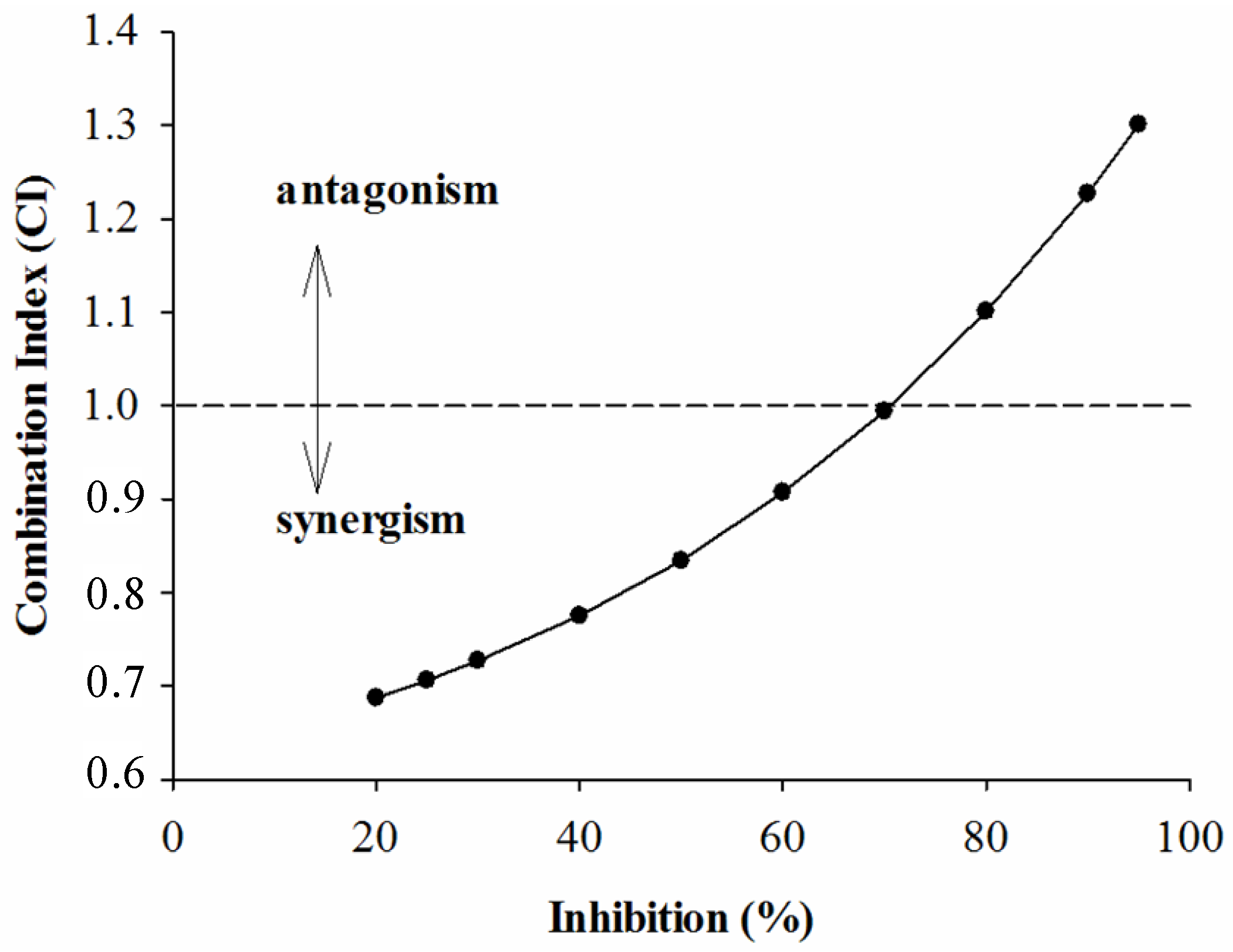
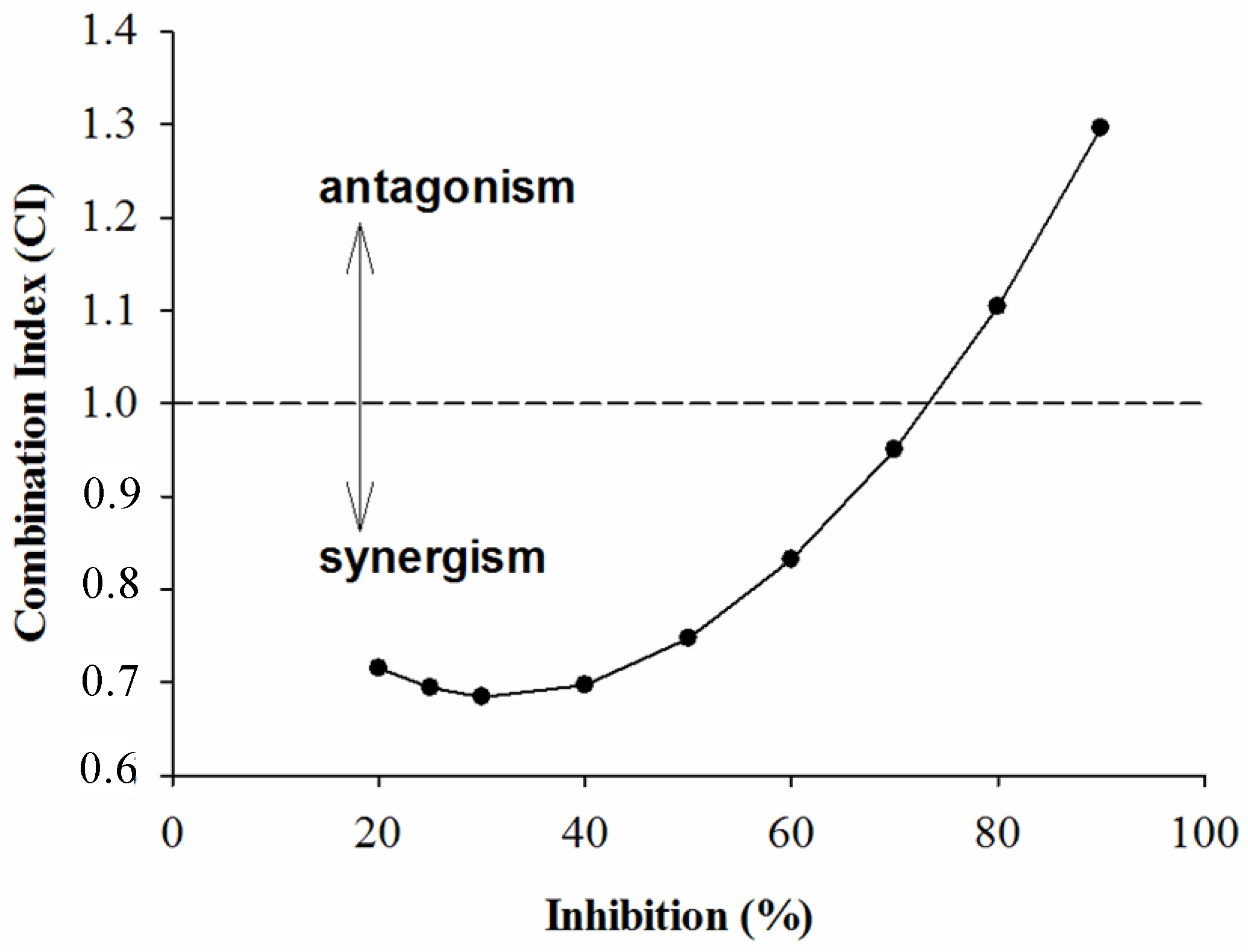

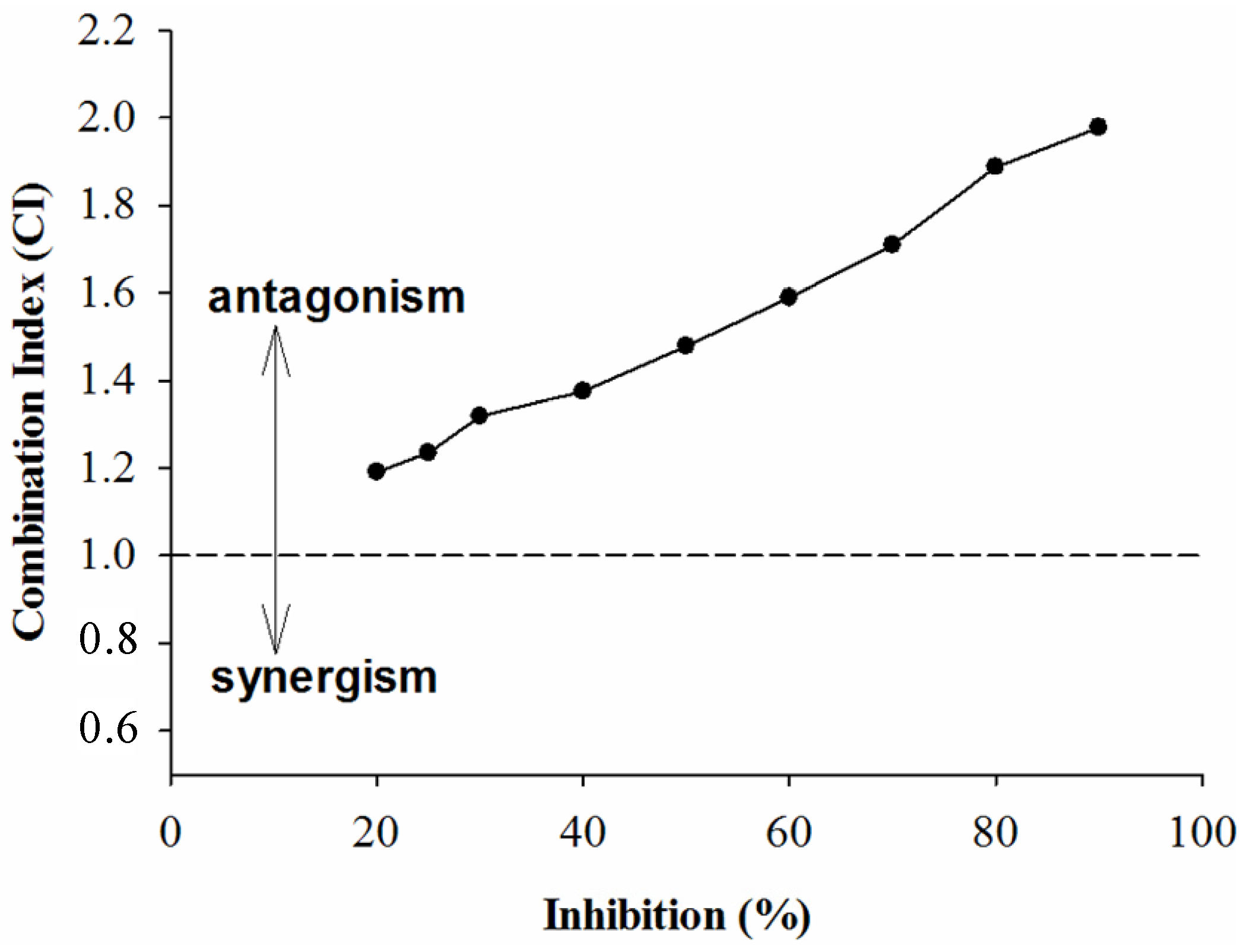
3. Experimental
| Samples | GA | GC | EGC | C | CAFF | EC | EGCG | GCG | ECG | CG |
|---|---|---|---|---|---|---|---|---|---|---|
| GTE | ND | 10.89% | 15.97% | 1.66% | 10.91% | 11.75% | 28.94% | 1.51% | 14.50% | 3.84% |
| GTP | ND | ND | ND | 1.07% | ND | 8.75% | 68.01% | 4.91% | ND | 16.67% |
| EGCG | ND | ND | ND | 3.38% | ND | ND | 95.62% | ND | ND | 1.01% |
3.1. α-Glucosidase Inhibitory Activity
3.2. α-Amylase Inhibition Activity
3.3. Experimental Design of Combination Study
3.4. Mathematical Analysis

3.5. Statistic Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food. Chem. 2009, 58, 770–774. [Google Scholar]
- Kim, J.S.; Hyun, T.K.; Kim, M.J. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011, 124, 1647–1651. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food. Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Jurgoński, A.; Brzuzan, Ł.; Godycka-Kłos, I.; Żary-Sikorska, E. Extract of green tea leaves partially attenuates streptozotocin-induced changes in antioxidant status and gastrointestinal functioning in rats. Nutr. Res. 2008, 28, 343–349. [Google Scholar] [CrossRef]
- Polychronopoulos, E.; Zeimbekis, A.; Kastorini, C.M.; Papairakleous, N.; Vlachou, I.; Bountziouka, V.; Panagiotakos, D.B. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study). Eur. J. Nutr. 2008, 47, 10–16. [Google Scholar] [CrossRef]
- Kuttan, R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food. Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef]
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef]
- Kamiyama, O.; Sanae, F.; Ikeda, K.; Higashi, Y.; Minami, Y.; Asano, N.; Adachi, I.; Kato, A. In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010, 122, 1061–1066. [Google Scholar] [CrossRef]
- Bischoff, H.; Puls, W.; Krause, H.; Schutt, H.; Thomas, G. Pharmacological properties of the novel glucosidase inhibitors BAY m 1099 (miglitol) and BAY o 1248. Diabetes Res. Clin. Pract. 1985, 1, 53. [Google Scholar]
- Horii, S.; Fukase, H.; Matsuo, T.; Kameda, Y.; Asano, N.; Matsui, K. Synthesis and. alpha.-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J. Med. Chem. 1986, 29, 1038–1046. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In vitro Inhibitory effects of cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Shao, S.; Qian, L.; Xu, P. Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: Antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind. Crops Prod. 2012, 37, 520–526. [Google Scholar] [CrossRef]
- Chou, T.C.; Motzer, R.J.; Tong, Y.; Bosl, G.J. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J. Natl. Cancer I. 1994, 86, 1517–1524. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, J.; Xu, P.; Wang, Y.; Wang, Y.; Hochstetter, D. Combined Effects of Green Tea Extracts, Green Tea Polyphenols or Epigallocatechin Gallate with Acarbose on Inhibition against α-Amylase and α-Glucosidase in Vitro. Molecules 2013, 18, 11614-11623. https://doi.org/10.3390/molecules180911614
Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined Effects of Green Tea Extracts, Green Tea Polyphenols or Epigallocatechin Gallate with Acarbose on Inhibition against α-Amylase and α-Glucosidase in Vitro. Molecules. 2013; 18(9):11614-11623. https://doi.org/10.3390/molecules180911614
Chicago/Turabian StyleGao, Junjie, Ping Xu, Yuefei Wang, Yiqi Wang, and Danielle Hochstetter. 2013. "Combined Effects of Green Tea Extracts, Green Tea Polyphenols or Epigallocatechin Gallate with Acarbose on Inhibition against α-Amylase and α-Glucosidase in Vitro" Molecules 18, no. 9: 11614-11623. https://doi.org/10.3390/molecules180911614