Bioactivity-Guided Fractionation of Physical Fatigue-Attenuating Components from Rubus parvifolius L.
Abstract
:1. Introduction
2. Results and Discussion
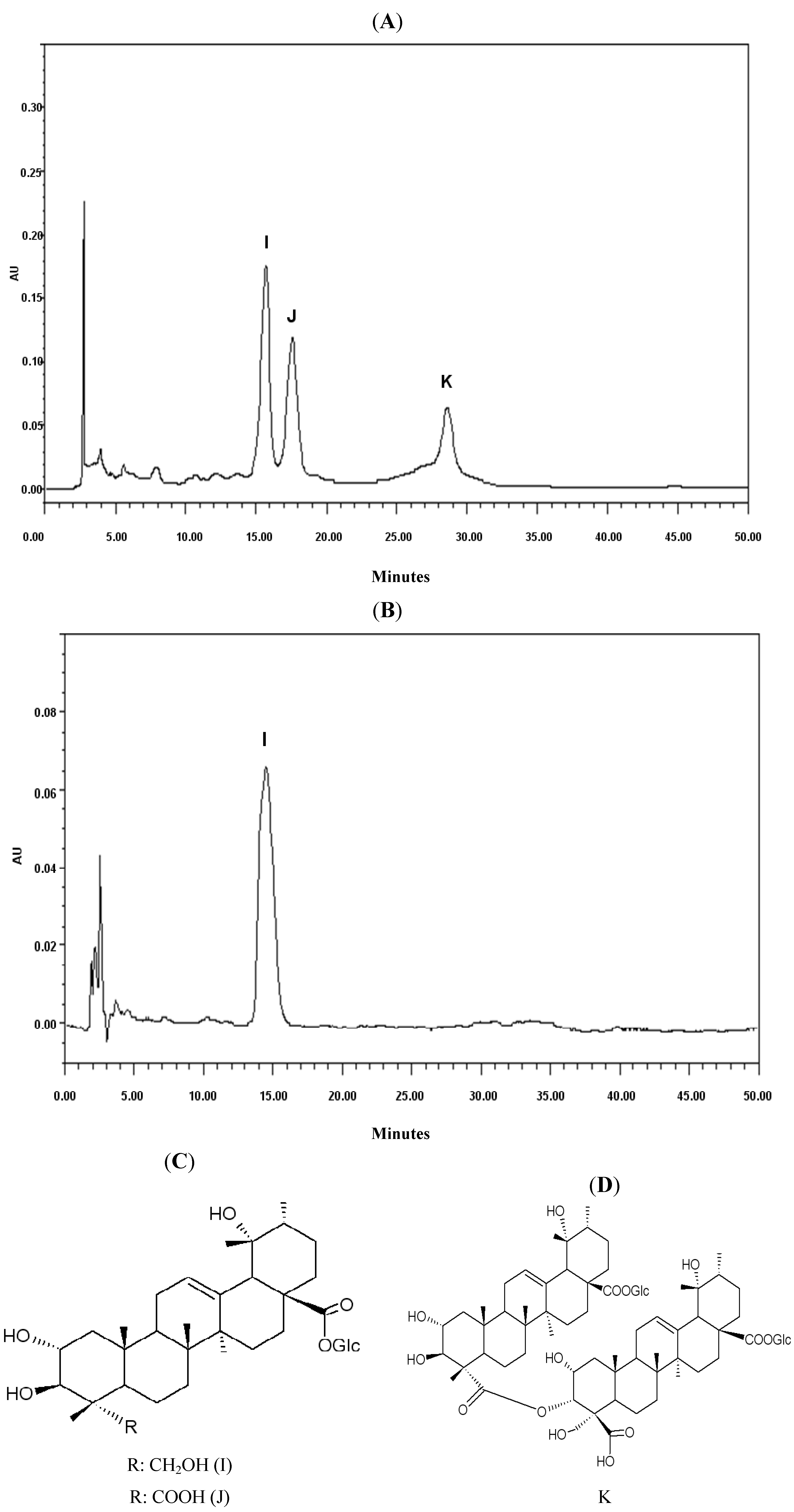
2.1. Chemical Analysis
2.2. The Effects of RPL on Mouse Body Weight Change
| Groups | Body weights(g) | ||
|---|---|---|---|
| Initial | Final | Increased | |
| Control | 23.8 ± 0.5 | 40.2 ± 0.8 | 16.4 ± 0.8 |
| Red Ginseng | 24.0 ± 0.7 | 39.6 ± 0.8 | 15.6 ± 0.9 |
| A1 | 24.4 ± 1.0 | 40.5 ± 0.9 | 16.1 ± 1.2 |
| A2 | 23.7 ± 0.9 | 39.6 ± 1.3 | 15.9 ± 1.2 |
| B1 | 24.2 ± 0.9 | 39.8 ± 1.3 | 15.6 ± 1.0 |
| B2 | 23.6 ± 0.9 | 39.3 ± 1.1 | 15.7 ± 0.8 |
| C1 | 24.2 ± 1.0 | 40.6 ± 1.2 | 16.3 ± 0.9 |
| C2 | 23.3 ± 0.9 | 39.5 ± 1.2 | 16.1 ± 1.4 |
| D1 | 24.5 ± 1.0 | 41.1 ± 1.3 | 16.6 ± 1.1 |
| D2 | 24.1 ± 0.8 | 39.9 ± 1.2 | 15.8 ± 1.2 |
| Groups | Body weights(g) | ||
|---|---|---|---|
| Initial | Final | Increased | |
| Normal | 23.2 ± 0.6 | 31.2 ± 0.8 | 8.1 ± 0.7 |
| Control | 23.3 ± 0.5 | 31.3 ± 0.6 | 8.0 ± 0.6 |
| RG1 | 23.8 ± 0.8 | 31.2 ± 0.7 | 7.4 ± 0.8 |
| RG2 | 24.0 ± 0.7 | 31.9 ± 0.6 | 7.9 ± 0.8 |
| E1 | 23.7 ± 0.9 | 31.6 ± 0.9 | 7.9 ± 0.7 |
| E2 | 23.9 ± 0.9 | 31.6 ± 0.7 | 7.7 ± 1.0 |
| F1 | 22.8 ± 0.8 | 31.2 ± 0.6 | 8.4 ± 1.1 |
| F2 | 23.5 ± 0.7 | 30.9 ± 0.6 | 7.4 ± 0.7 |
| G1 | 23.7 ± 0.9 | 31.0 ± 1.0 | 7.3 ± 0.8 |
| G2 | 23.2 ± 0.8 | 30.9 ± 0.9 | 7.7 ± 0.9 |
| H1 | 23.1 ± 0.8 | 30.6 ± 0.8 | 7.6 ± 0.7 |
| H2 | 22.9 ± 0.8 | 30.8 ± 0.9 | 7.9 ± 1.1 |
| I1 | 24.0 ± 1.0 | 32.1 ± 1.0 | 8.1 ± 0.9 |
| I2 | 23.7 ± 0.8 | 31.1 ± 0.9 | 7.5 ± 0.9 |
2.3. The Effects of RPL on Mice in Swimming-to-Exhaustion Test

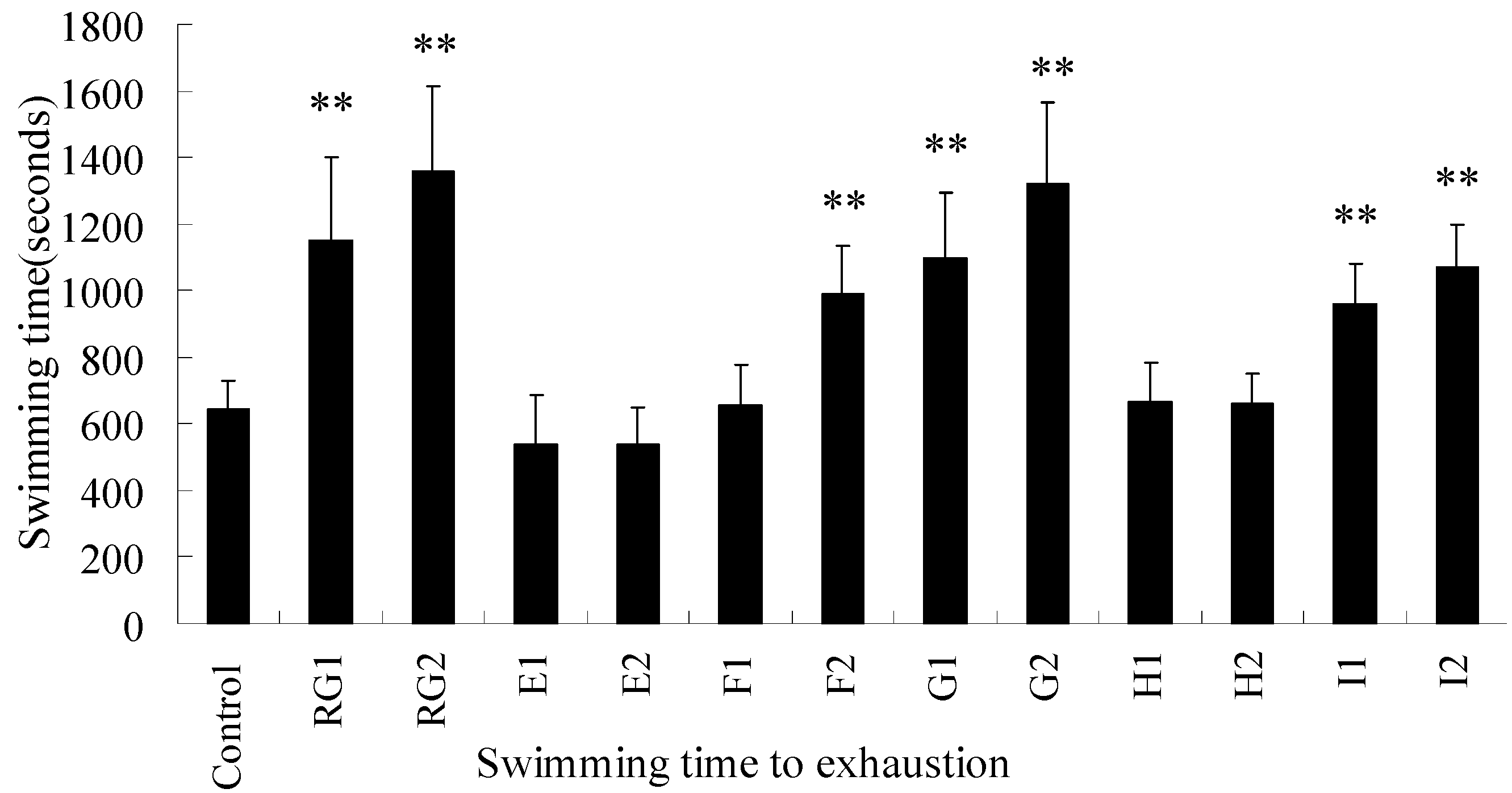
2.4. The Effects of RPL on SUN, TG, LDH, Ammonia, LA and HG
| Groups | SUN (mmol/L) | TG (mmol/L) | LDH (IU/L) | Ammonia (umol/L) |
|---|---|---|---|---|
| Control | 10.33 ± 0.63 | 2.880 ± 0.1052 | 585.1 ± 21.12 | 93.1 ± 7.29 |
| Red Ginseng | 9.29 ± 0.71 ** | 2.765 ± 0.1246 * | 590.7 ± 17.22 | 91.9 ± 6.71 |
| A1 | 9.95 ± 0.77 | 2.875 ± 0.0664 | 575.2 ± 23.64 | 94.9 ± 8.48 |
| A2 | 9.74 ± 0.76 | 2.858 ± 0.1055 | 580.8 ± 21.80 | 98.3 ± 8.51 |
| B1 | 9.81 ± 0.78 | 2.931 ± 0.1064 | 594.6 ± 28.41 | 87.2 ± 7.21 |
| B2 | 9.04 ± 0.66 * | 2.784 ± 0.0763 * | 587.7 ± 28.23 | 90.5 ± 6.38 |
| C1 | 9.41 ± 0.76 ** | 2.769 ± 0.0568 * | 597.2 ± 21.50 | 88.1 ± 6.67 |
| C2 | 8.84 ± 0.70 ** | 2.745 ± 0.0573 ** | 603.3 ± 27.45 | 85.6 ± 8.14 * |
| D1 | 9.72 ± 0.68 | 2.967 ± 0.0934 | 567.6 ± 23.28 | 94.7 ± 9.03 |
| D2 | 9.55 ± 0.69 * | 2.909 ± 0.1184 | 578.4 ± 24.91 | 95.2 ± 6.56 |
| Groups | BUN (mmol/L) | TG (mmol/L) | LDH (IU/L) | Ammonia (umol/L) | LA (mg /dL) | HG (mg/g) |
|---|---|---|---|---|---|---|
| Normal | 6.48 ± 0.45 | 1.959 ± 0.069 | 383.9 ± 23.27 | 52.2 ± 5.09 | 44.1 ± 4.6 | 47.90 ± 5.83 |
| Control | 9.66 ± 0.68 | 2.162 ± 0.083 | 894.5 ± 35.59 | 134.1 ± 7.26 | 65.9 ± 6.1 | 19.44 ± 1.96 |
| RG1 | 9.06 ± 0.86 | 2.077 ± 0.090 * | 919.9 ± 60.71 | 128.9 ± 10.21 | 61.3 ± 5.6 | 39.26 ± 3.82 ** |
| RG2 | 8.91 ± 0.71 * | 2.034 ± 0.077 ** | 922.4 ± 49.74 | 127.4 ± 9.62 | 59.3 ± 6.4 * | 43.17 ± 5.38 ** |
| E1 | 9.38 ± 0.70 | 2.148 ± 0.077 | 927.5 ± 50.40 | 129.7 ± 9.32 | 62.2 ± 6.9 | 19.76 ± 1.93 |
| E2 | 9.66 ± 0.84 | 2.134 ± 0.053 | 921.1 ± 43.57 | 127.7 ± 12.56 | 61.9 ± 8.7 | 20.20 ± 1.72 |
| F1 | 9.41 ± 0.63 | 2.083 ± 0.123 | 908.3 ± 38.90 | 126.2 ± 10.50 | 64.6 ± 8.8 | 20.60 ± 2.47 |
| F2 | 9.21 ± 0.75 | 2.052 ± 0.092 * | 932.5 ± 42.57 * | 121.1 ± 15.02 * | 63.8 ± 8.3 | 19.30 ± 2.06 |
| G1 | 8.90 ± 0.71 * | 2.050 ± 0.096 * | 938.2 ± 47.62 * | 115.7 ± 12.94 ** | 57.8 ± 7.1 * | 40.59 ± 4.19 ** |
| G2 | 8.58 ± 0.69 ** | 1.992 ± 0.074 ** | 970.5 ± 81.40 * | 104.1 ± 11.62 ** | 54.5 ± 6.3 ** | 43.04 ± 4.59 ** |
| H1 | 9.30 ± 0.82 | 2.165 ± 0.104 | 903.2 ± 33.03 | 125.7 ± 14.64 | 63.4 ± 8.1 | 21.58 ± 3.34 |
| H2 | 9.14 ± 0.98 | 2.178 ± 0.125 | 916.2 ± 30.97 | 135.1 ± 11.28 | 62.2 ± 7.1 | 21.89 ± 3.60 |
| I1 | 9.09 ± 0.83 | 2.170 ± 0.103 | 937.0 ± 44.94 * | 113.3 ± 11.54 ** | 60.9 ± 4.3 * | 38.67 ± 3.24 ** |
| I2 | 8.79 ± 0.76 * | 2.067 ± 0.107 * | 951.9 ± 58.00 * | 109.0 ± 15.19 ** | 58.7 ± 5.7 * | 41.45 ± 3.81 ** |
2.5. The Effects of RPL on IL-6 and TNF-α
| Groups | IL-6 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|
| Normal | 224.19 ± 19.51 ** | 870.08 ± 53.65 ** |
| Control | 348.04 ± 25.39 | 978.43 ± 52.14 |
| RG1 | 328.97 ± 17.46 | 926.13 ± 34.58 * |
| RG2 | 320.11 ± 19.94 * | 905.50 ± 44.7 ** |
| E1 | 334.61 ± 26.80 | 941.72 ± 47.44 |
| E2 | 340.64 ± 18.67 | 945.96 ± 73.91 |
| F1 | 328.69 ± 30.19 | 956.67 ± 53.94 |
| F2 | 339.75 ± 26.34 | 945.39 ± 75.32 |
| G1 | 317.34 ± 29.03 * | 912.73 ± 35.94 ** |
| G2 | 310.84 ± 32.74 * | 907.71 ± 39.16 ** |
| H1 | 337.22 ± 34.54 | 937.43 ± 45.74 |
| H2 | 343.11 ± 33.55 | 947.29 ± 49.97 |
| I1 | 325.76 ± 19.94 * | 931.77 ± 38.32 * |
| I2 | 324.21 ± 17.17 * | 923.91 ± 44.69 * |
3. Experimental
3.1. Chemicals, Reagents and Instruments
3.2. Plant Materials
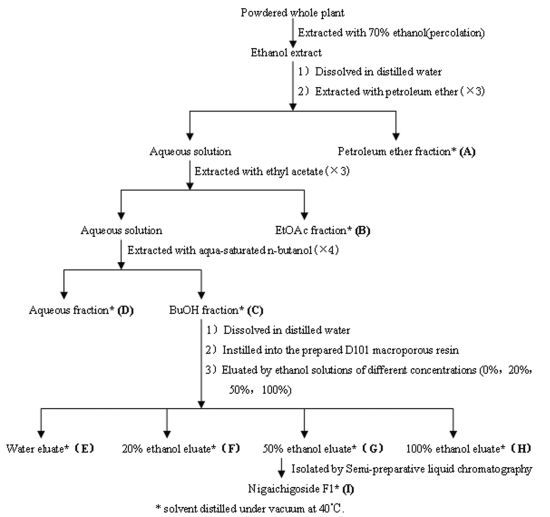
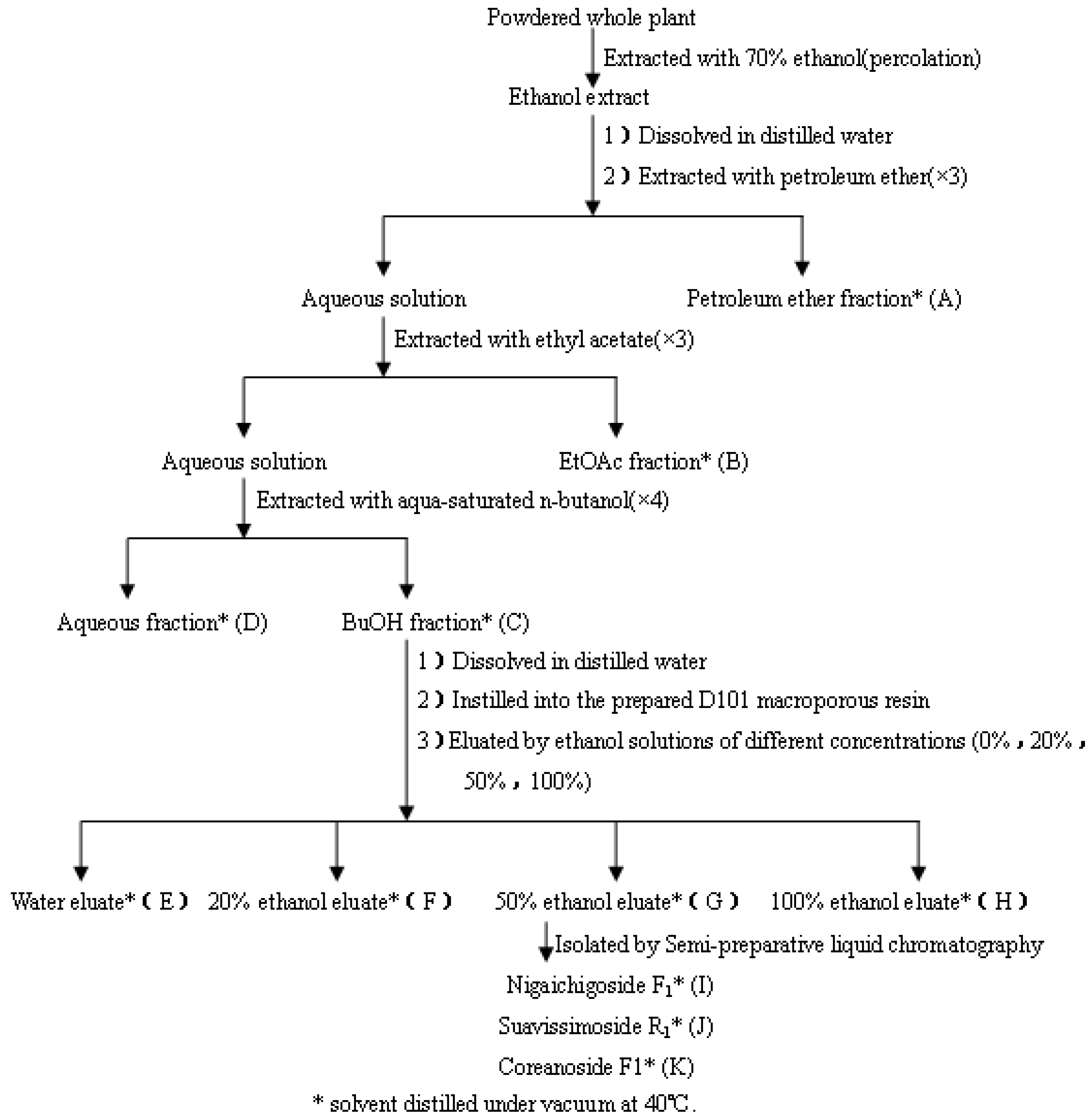
3.3. Extraction, Isolation of RPL Components
3.4. Animals
3.5. Experimental Design
3.5.1. Weight-Loaded Swimming Experiment I
3.5.2. Analysis of Blood Biochemical Parameters
3.5.3. Weight-Loaded Swimming Experiment II
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Compilation Group of Chinese Herbal Medicine Compilation. Chinese Herbal Medicine Compilation; People’s Medical Publishing House: Beijing, China, 1975; p. 512. [Google Scholar]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Shyur, L.F.; Tsung, J.H.; Chiu, C.Y.; Lo, C.P. Antioxidant properties of extracts from medicinal plants popularly used inTaiwan. Int. J. Appl. Sci. Eng. 2005, 3, 195–202. [Google Scholar]
- Gao, J.; Sun, C.R.; Yang, J.H.; Shi, J.M.; Du, Y.G.; Zhang, Y.Y.; Li, J.H.; Wan, H.T. Evaluation of the hepatoprotective and antioxidant activities of Rubus parvifolius L. J. Zhejiang Univ. Sci. B 2011, 12, 135–142. [Google Scholar] [CrossRef]
- Wang, J.S.; Li, H.Z.; Qiu, Z.Y.; Xia, Y.P.; Ren, L.Y.; Zhou, C.L. Protective effects of aqueous extract of Rubus parviflolius on middle cerebral artery occlusion and reperfusion injury in rats. Chin. J. New Drugs Clin. Remed. 2006, 12, 920–923. [Google Scholar]
- Wang, J.S.; Qiu, Z.Y.; Xia, Y.P.; Li, H.Z.; Ren, L.Y.; Zhang, L. The protective effects of total glycosides Rubus parviflolius on cerebral ischemia in rat. Zhongguo Zhong Yao Za Zhi. 2006, 2, 138–141. [Google Scholar]
- Zheng, Z.X.; Zhang, L.J.; Hang, C.X. Antitumor effects of the total saponins of Rubus parvifolius L. on malignant melanoma. Zhongguo Zhong Yao Za Zhi. 2007, 32, 2055–2058. [Google Scholar]
- Akazawa, K.H.; Cui, Y.; Tanaka, M.; Kataoka, Y.; Yoneda, Y.; Watanabe, Y. Mapping of regional brain activation in response to fatigue-load and recovery in rats with c-Fos immunohistochemistry. Neurosci. Res. 2009, 66, 372–379. [Google Scholar]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Huang, L.Z.; Huang, B.K.; Ye, Q.; Qin, L.P. Bioactivity-guided fractionation for anti-fatigue property of Acanthopanax senticosus. J. Ethnopharmacol. 2011, 133, 213–219. [Google Scholar] [CrossRef]
- Seto, T.; Tanaka, T.; Tanaka, O.; Naruhashi, N. β-glucosyl esters of 19α-hydroxyursolic acid derivatives in leaves of Rubus species. Phytochemistry 1984, 23, 2829–2834. [Google Scholar] [CrossRef]
- Du, S.H.; Liu, W.Y.; Rao, J.H.; Bai, J. Two isolated saponins from Rubus parvifolius L. on preparative HPLC. Chin. Tradit. Herbal Drugs 2005, 36, 348–350. [Google Scholar]
- Nguelefack, T.B.; Mbakam, F.H.K.; Tapondjou, L.A.; Watcho, P.; Nguelefack-Mbuyo, E.P.; Ponou, B.K.; Kamanyi, A.; Park, H.-J. A dimeric triterpenoid glycoside and flavonoid glycosides with free radical-scavenging activity isolated from rubus rigidus var. camerunensis. Arch. Pharm. Res. 2011, 34, 543–550. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.S.; Fan, Y.Y.; Chen, Y.; Liu, D.; Cheng, H.R.; Gao, X.G. Anti-fatigue activity of the water-soluble polysaccharides isolated from panax ginseng C. A. Meyer. J. Ethnopharmacol. 2010, 130, 421–423. [Google Scholar] [CrossRef]
- Huang, L.Z.; Huang, B.K.; Liang, J.; Zheng, C.J.; Han, T.; Zhang, Q.Y.; Qin, L.P. Antifatigue activity of the liposoluble fraction from Acanthopanax senticosus. Phytochem. Res. 2011, 25, 940–943. [Google Scholar]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabàk, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical activity and inflammatory markers over 10 years follow-up in men and women from the whitehall II cohort study. Exerc. Physiol. 2012, 126, 928–933. [Google Scholar]
- Cho, H.J.; Kivimaki, M.; Bower, J.E.; Irwin, M.R. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol. Med. 2013, 43, 1773–1783. [Google Scholar] [CrossRef]
- Sun, R.; Fu, K.; Fu, Y.; Zu, Y.; Wang, Y.; Luo, M.; Li, S.; Luo, H.; Li, Z. Preparative separation and enrichment of four taxoids fromTaxus chinensis needles extracts by macroporous resin column chromatography. J. Sep. Sci. 2009, 32, 1284–1293. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Gao, J.; Ding, X.; Gao, S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 inmice by intranasally administration. Biol. Phar.-Maceutical. Bull. 2008, 31, 2024–2027. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, X.; Bao, B. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 2006, 20, 872–876. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, L.D.; Xia, X.; Kung, H.F.; Zhang, L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J. Ethnopharmacol. 2005, 96, 451–459. [Google Scholar] [CrossRef]
- Xu, L.R.; Wang, J.S.; Li, H.Z. Effects of Rubus parvifolius L. on neuronal apoptosis and expression of apoptosis-related proteins in a rat model of focal cerebral ischemic-reperfusion injury. Neural Regen. Res. 2008, 3, 742–746. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Wang, X.; Cai, Y.; Tang, M.; Dai, Q.; Hu, X.; Huang, M.; Sun, F.; Liu, Y.; Xia, P. Bioactivity-Guided Fractionation of Physical Fatigue-Attenuating Components from Rubus parvifolius L. Molecules 2013, 18, 11624-11638. https://doi.org/10.3390/molecules180911624
Chen J, Wang X, Cai Y, Tang M, Dai Q, Hu X, Huang M, Sun F, Liu Y, Xia P. Bioactivity-Guided Fractionation of Physical Fatigue-Attenuating Components from Rubus parvifolius L. Molecules. 2013; 18(9):11624-11638. https://doi.org/10.3390/molecules180911624
Chicago/Turabian StyleChen, Jianhong, Xianfeng Wang, Yongqing Cai, Ming Tang, Qing Dai, Xiaogang Hu, Mingchun Huang, Fengjun Sun, Yao Liu, and Peiyuan Xia. 2013. "Bioactivity-Guided Fractionation of Physical Fatigue-Attenuating Components from Rubus parvifolius L." Molecules 18, no. 9: 11624-11638. https://doi.org/10.3390/molecules180911624