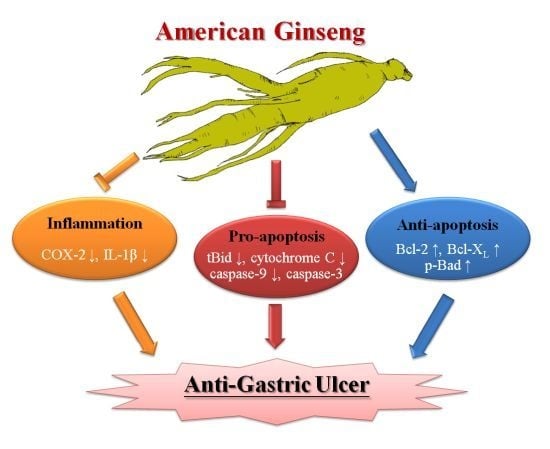
Cytoprotective Effect of American Ginseng in a Rat Ethanol Gastric Ulcer Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight

2.2. Effect of AG Supplementation on Ethanol-induced Gastric Mucosal Damage

2.3. Histology

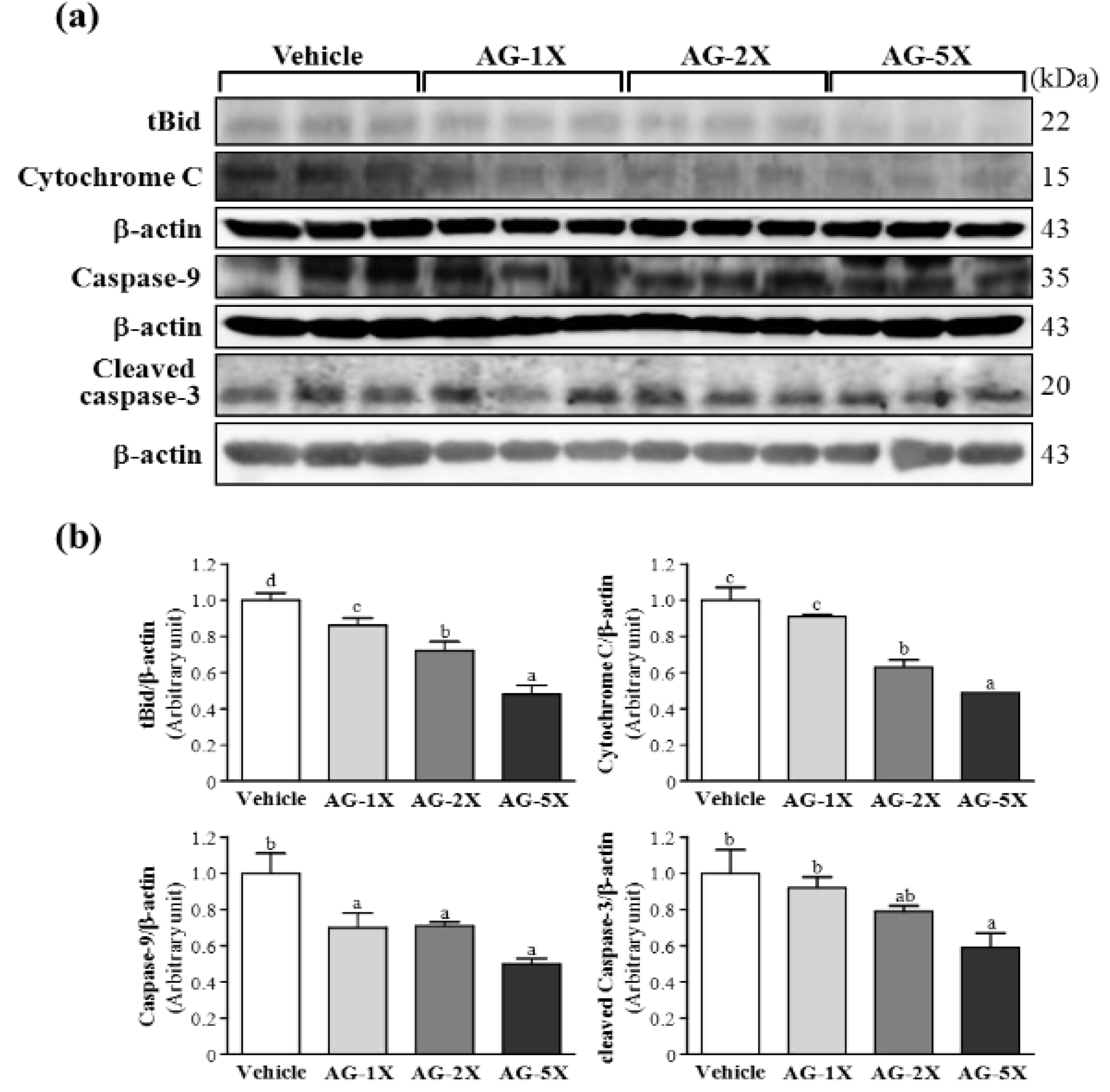
2.5. Effect of AG Supplementation on Ethanol-induced Expression of Apoptosis Proteins in Rat Stomachs
2.6. Effect of AG Supplementation on Expression of Anti-Apoptosis Proteins in Rat Stomachs

3. Experimental
3.1. Chemicals and Antibodies
3.2. Induction of Gastric Ulcer and Histology
3.3. Effect of AG Supplementation on Ethanol-Induced Gastric Mucosal Damage
3.4. Western Blot Analysis
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. American ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: A randomized, double-blind trial, N07C2. J. Natl. Cancer Inst. 2013, 105, 1230–1238. [Google Scholar] [CrossRef]
- Lemmon, H.R.; Sham, J.; Chau, L.A.; Madrenas, J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: Characterization of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 2012, 142, 1–13. [Google Scholar] [CrossRef]
- Rai, D.; Bhatia, G.; Sen, T.; Palit, G. Anti-stress effects of Ginkgo biloba and Panax ginseng: A comparative study. J. Pharmacol. Sci. 2003, 93, 458–464. [Google Scholar] [CrossRef]
- Sen, S.; Querques, M.A.; Chakrabarti, S. North American Ginseng (Panax quinquefolius) prevents hyperglycemia and associated pancreatic abnormalities in diabetes. J. Med. Food. 2013, 16, 587–592. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y. Current evaluation of the millennium phytomedicine--ginseng (I): Etymology, pharmacognosy, Phytochemistry, Market and regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef]
- Qu, C.L.; Bai, Y.P.; Jin, X.Q.; Wang, Y.T.; Zhang, K.; You, J.Y. Study on ginsenosides in different parts and ages of Panax. quinquefolius L. Food Chem. 2009, 115, 340–346. [Google Scholar] [CrossRef]
- Lim, W.; Mudge, K.W.; Vermeylen, F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax. quinquefolium). J. Agric. Food Chem. 2005, 53, 8498–8505. [Google Scholar] [CrossRef]
- Kang, K.S.; Yamabe, N.; Kim, H.Y.; Okamoto, T.; Sei, Y.; Yokozawa, T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J. Ethnopharmacol. 2007, 113, 225–232. [Google Scholar] [CrossRef]
- AlRashdi, A.S.; Salama, S.M.; Alkiyumi, S.S.; Abdulla, M.A.; Hadi, A.H.A.; Abdelwahab, S.I.; Taha, M.M.; Hussiani, J.; Asykin, N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum. sambac against HCl/Ethanol-Induced gastric mucosal injury in rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 786426. [Google Scholar]
- Kauffman, G.L., Jr. The gastric mucosal barrier. Component control. Dig. Dis. Sci. 1985, 30, 69S–76S. [Google Scholar] [CrossRef]
- Dai, J.; Lin, D.; Zhang, J.; Habib, P.; Smith, P.; Murtha, J.; Fu, Z.; Yao, Z.; Qi, Y.; Keller, E.T. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. Clin. Invest. 2000, 106, 887–895. [Google Scholar]
- Hwang, I.R.; Kodama, T.; Kikuchi, S.; Sakai, K.; Peterson, L.E.; Graham, D.Y.; Yamaoka, Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1β production in Helicobacter pylori infection. Gastroenterology 2002, 123, 1793–1803. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kita, M.; Kodama, T.; Sawai, N.; Kashima, K.; Imanishi, J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 1997, 41, 442–451. [Google Scholar] [CrossRef]
- Tanaka, A.; Araki, H.; Komoike, Y.; Hase, S.; Takeuchi, K. Inhibition of both COX-1 and COX-2 is required for development of gastric damage in response to nonsteroidal antiinflammatory drugs. J. Physiol. Paris 2001, 95, 21–27. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Hegde, R.; Srinivasula, S.M.; Zhang, Z.J.; Wassell, R.; Mukattash, R.; Cilenti, L.; DuBois, G.; Lazebnik, Y.; Zervos, A.S.; Fernandes-Alnemri, T.; et al. Identification of omi/htra2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 2002, 277, 432–438. [Google Scholar]
- Hu, C.; Song, G.; Zhang, B.; Liu, Z.; Chen, R.; Zhang, H.; Hu, T. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via Bid-mediated mitochondrial pathway. J. Cell. Mol. Med. 2012, 16, 96–106. [Google Scholar] [CrossRef]
- Luo, J.Z.; Luo, L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured β cells. Evid. Based Complement. Alternat. Med. 2006, 3, 365–372. [Google Scholar] [CrossRef]
- Liu, W.K.; Xu, S.X.; Che, C.T. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef]
- Tudor, G.; Aguilera, A.; Halverson, D.O.; Laing, N.D.; Sausville, E.A. Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000, 7, 574–586. [Google Scholar]
- Lei, K.; Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 2003, 100, 2432–2437. [Google Scholar] [CrossRef]
- Newmeyer, D.D.; Bossy-Wetzel, E.; Kluck, R.M.; Wolf, B.B.; Beere, H.M.; Green, D.R. Bcl-xL does not inhibit the function of Apaf-1. Cell Death Differ. 2000, 7, 402–407. [Google Scholar] [CrossRef]
- Park, S.; Shin, W.S.; Ho, J. Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice. J. Ethnopharmacol. 2011, 138, 340–344. [Google Scholar] [CrossRef]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Shay, H.; Komarov, S.A.; Fels, S.S.; Meranze, D.; Gruenstein, M.; Siplet, H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 1945, 5, 43–61. [Google Scholar]
- Haule, E.E.; Moshi, M.J.; Nondo, R.S.; Mwangomo, D.T.; Mahunnah, R.L. A study of antimicrobial activity, Acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCl gastric ulcer model. BMC Res. Notes 2012, 5, 546. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Chiang, W.D.; Huang, W.C.; Huang, C.Y.; Hsu, M.C.; Lin, W.T. Hepatoprotective effects of swimming exercise against D-galactose-induced senescence rat model. Evid. Based Complement. Alternat. Med. 2013, 2013, 275431. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, C.-C.; Chen, Y.-M.; Wang, D.-C.; Chiu, C.-C.; Lin, W.-T.; Huang, C.-Y.; Hsu, M.-C. Cytoprotective Effect of American Ginseng in a Rat Ethanol Gastric Ulcer Model. Molecules 2014, 19, 316-326. https://doi.org/10.3390/molecules19010316
Huang C-C, Chen Y-M, Wang D-C, Chiu C-C, Lin W-T, Huang C-Y, Hsu M-C. Cytoprotective Effect of American Ginseng in a Rat Ethanol Gastric Ulcer Model. Molecules. 2014; 19(1):316-326. https://doi.org/10.3390/molecules19010316
Chicago/Turabian StyleHuang, Chi-Chang, Yi-Ming Chen, Dean-Chuan Wang, Chien-Chao Chiu, Wan-Teng Lin, Chih-Yang Huang, and Mei-Chich Hsu. 2014. "Cytoprotective Effect of American Ginseng in a Rat Ethanol Gastric Ulcer Model" Molecules 19, no. 1: 316-326. https://doi.org/10.3390/molecules19010316