Preventive Effects of Citrus unshiu Peel Extracts on Bone and Lipid Metabolism in OVX Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Hesperidin from Dried Citrus unshiu Peel Extracts

2.2. Effect of DCPE on Body Weight and Serum Lipid Concentrations in OVX Rats

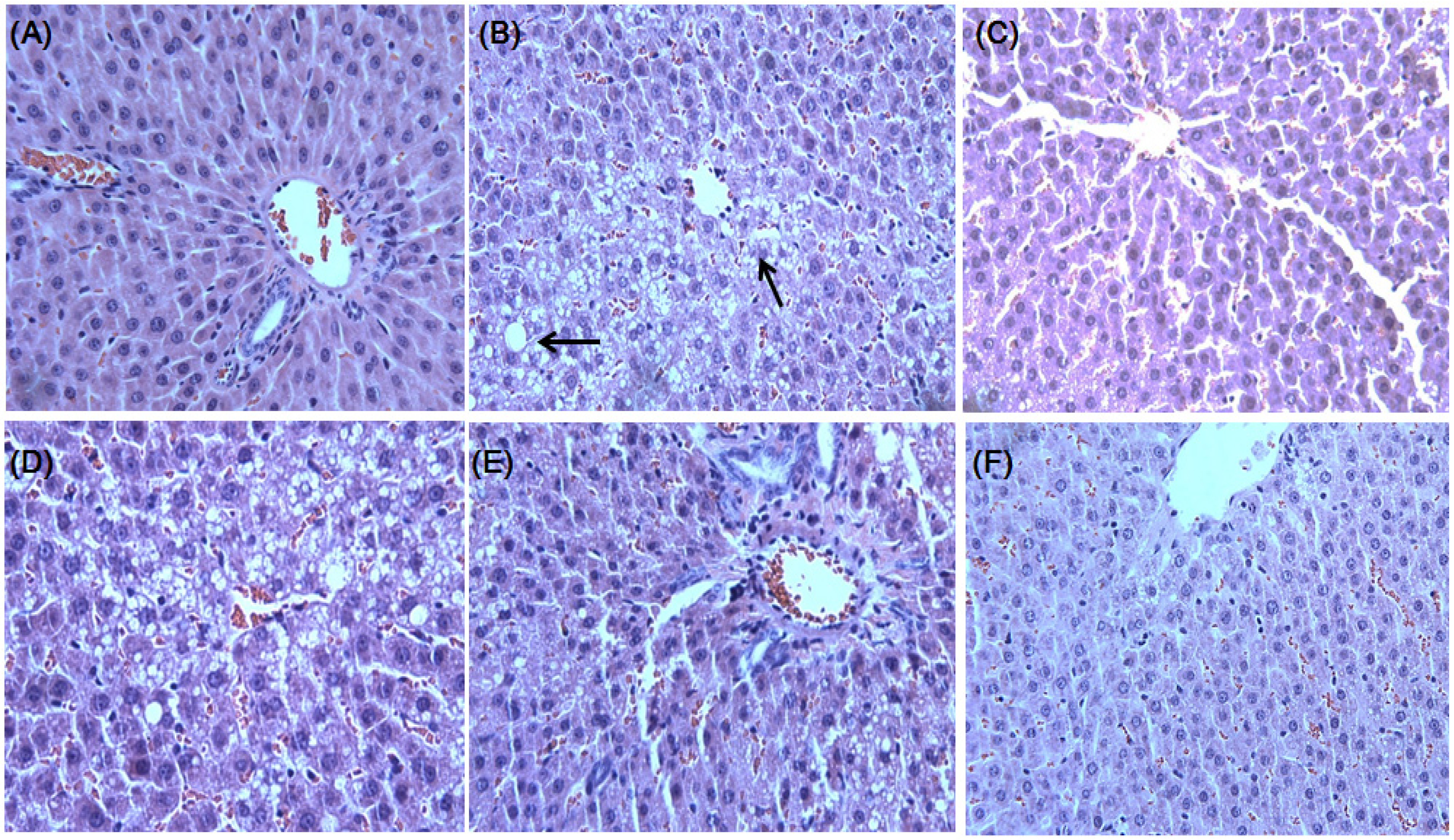
2.3. Effect of DCPE on OVX-Induced Fatty Liver
| Groups | TC | TG | HDL-c | LDL-c | CRI | AI |
|---|---|---|---|---|---|---|
| (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | |||
| I | 75.8 ± 8.5 | 78.5 ± 5.2 | 50.5 ± 4.1 | 15.7 ± 6.1 | 1.7 ± 0.2 | 0.3 ± 0.2 |
| II | 97.4 ± 5.1 c | 98.3 ± 6.4 c | 41.5 ± 2.3 | 33.6 ± 4.2 c | 2.3 ± 0.3 c | 0.8 ± 0.2 c |
| III | 90.0 ± 4.8 | 85.7 ± 8.7 a | 44.8 ± 4.3 | 18.3 ± 5.4 a | 2.0 ± 0.2 a | 0.4 ± 0.1 a |
| IV | 98.2 ± 5.8 | 90.2 ± 5.4 | 42.5 ± 3.8 | 27.3 ± 4.2 | 2.3 ± 0.3 | 0.6 ± 0.1 |
| V | 92.0 ± 5.4 | 88.7 ± 8.7 | 42.5 ± 4.8 | 26.3 ± 5.4 | 2.2 ± 0.2 | 0.6 ± 0.2 |
| VI | 85.0 ± 6.5 a | 80.9 ± 5.6 a | 48.9 ± 4.6 | 17.9 ± 4.8 a | 1.7 ± 0.2 a | 0.4 ± 0.3 b |
| Groups | Serum (mg/dL) | Liver lipid (mg/g wet wt) | ||
|---|---|---|---|---|
| AST | ALT | TC | TG | |
| I | 115.1 ± 11.6 | 62.1 ± 6.9 | 6.1 ± 0.7 | 38.2 ± 5.9 |
| II | 144.4 ± 25.7 c | 96.5 ± 6.6 c | 10.1 ± 0.7 c | 69.8 ± 6.4 c |
| III | 121.2 ± 17.4 a | 72.5 ± 5.6 a | 7.0 ± 0.5 a | 54.5 ± 6.2 a |
| IV | 132.3 ± 16.4 | 84.5 ± 6.6 | 8.2 ± 0.6 | 70.5 ± 4.2 |
| V | 123.1 ± 16.4 a | 75.3 ± 4.9 a | 7.2 ± 0.6 a | 52.8 ± 6.8 a |
| VI | 118.6 ± 10.5 a | 67.5 ± 6.8 a | 6.5 ± 0.5 a | 44.1 ± 5.8 a |
2.4. Effect of DCPE on BMD and Organ Weights in OVX Rats

2.5. Effect of DCPE on Bone Marker in OVX Rats

2.6. Discussion
3. Experimental
3.1. Sample Preparation and HPLC Analysis
3.2. Animals and Treatments
3.3. BMD Measurements
3.4. Serum Lipid, Estradiol and Bone Marker Analysis
3.5. Liver Histological Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pinkerton, J.V.; Guico-Pabia, C.J.; Taylor, H.S. Menstrual cycle-related exacerbation of disease. Am. J. Obstet. Gynecol. 2010, 202, 221–231. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sowers, M. The menopause transition. Endocrinol. Metab. Clin. North Am. 1997, 26, 261–277. [Google Scholar] [CrossRef]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002, 288, 872–881. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, Hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women’s health initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar] [CrossRef]
- Hedelin, M.; Klint, A.; Chang, E.T.; Bellocco, R.; Johansson, J.E.; Andersson, S.O.; Heinonen, S.M.; Adlercreutz, H.; Adami, H.O.; Gronberg, H.; et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: The cancer prostate sweden study (sweden). Cancer Causes Control 2006, 17, 169–180. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and ldl cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: Meta-analysis of randomized controlled trials. Clin. Nutr. 2008, 27, 57–64. [Google Scholar] [CrossRef]
- Shedd-Wise, K.M.; Alekel, D.L.; Hofmann, H.; Hanson, K.B.; Schiferl, D.J.; Hanson, L.N.; van Loan, M.D. The soy isoflavones for reducing bone loss study: 3-yr effects on pqct bone mineral density and strength measures in postmenopausal women. J. Clin. Densitom. 2011, 14, 47–57. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Vatanparast, H.; Pierson, R.; Case, A.; Olatunbosun, O.; Whiting, S.J.; Beck, T.J.; Pahwa, P.; Biem, H.J. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: A randomized clinical trial. J. Bone Miner. Res. 2013, 28, 780–793. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Kim, M.H.; Ko, H.C.; Kim, S.J. Immature citrus sunki peel extract exhibits antiobesity effects by beta-oxidation and lipolysis in high-fat diet-induced obese mice. Biol. Pharm. Bull. 2012, 35, 223–230. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Oh, Y.C.; Cho, W.K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.H.; Ma, J.Y. Anti-inflammatory effect of citrus unshiu peel in lps-stimulated raw 264.7 macrophage cells. Am. J. Chin. Med. 2012, 40, 611–629. [Google Scholar]
- Kim, D.K.; Lee, K.T.; Eun, J.S.; Zee, O.P.; Lim, J.P.; Eum, S.S.; Kim, S.H.; Shin, T.Y. Anti-allergic components from the peels of citrus unshiu. Arch. Pharm. Res. 1999, 22, 642–645. [Google Scholar] [CrossRef]
- Yang, G.; Lee, J.; Jung, E.D.; Ham, I.; Choi, H.Y. Lipid lowering activity of citri unshii pericarpium in hyperlipemic rats. Immunopharmacol. Immunotoxicol. 2008, 30, 783–791. [Google Scholar] [CrossRef]
- Lee, S.; Ra, J.; Song, J.Y.; Gwak, C.; Kwon, H.J.; Yim, S.V.; Hong, S.P.; Kim, J.; Lee, K.H.; Cho, J.J.; et al. Extracts from citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J. Ethnopharmacol. 2011, 133, 973–979. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Bucheli, P.; Wei, D. Citrus flavonoids in fruit and traditional chinese medicinal food ingredients in china. Plant Food. Hum. Nutr. 2006, 61, 57–65. [Google Scholar]
- Bicu, I.; Mustata, F. Cellulose extraction from orange peel using sulfite digestion reagents. Bioresource Technol. 2011, 102, 10013–10019. [Google Scholar] [CrossRef]
- Deyhim, F.; Garica, K.; Lopez, E.; Gonzalez, J.; Ino, S.; Garcia, M.; Patil, B.S. Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 2006, 22, 559–563. [Google Scholar] [CrossRef]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal Interact. 2001, 1, 193–207. [Google Scholar]
- Lim, D.W.; Kim, J.G.; Lee, Y.; Cha, S.H.; Kim, Y.T. Preventive effects of eleutherococcus senticosus bark extract in ovx-induced osteoporosis in rats. Molecules 2013, 18, 7998–8008. [Google Scholar] [CrossRef]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaisse, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [Green Version]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Nishizawa, Y.; Nakamura, T.; Ohta, H.; Kushida, K.; Gorai, I.; Shiraki, M.; Fukunaga, M.; Hosoi, T.; Miki, T.; Chaki, O.; et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J. Bone Miner. Metab. 2005, 23, 97–104. [Google Scholar]
- Hertrampf, T.; Schleipen, B.; Offermanns, C.; Velders, M.; Laudenbach, U.; Diel, P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol. Lett. 2009, 184, 198–203. [Google Scholar] [CrossRef]
- Devareddy, L.; Khalil, D.A.; Smith, B.J.; Lucas, E.A.; Soung, D.Y.; Marlow, D.D.; Arjmandi, B.H. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone 2006, 38, 686–693. [Google Scholar] [CrossRef]
- Dang, Z.C.; van Bezooijen, R.L.; Karperien, M.; Papapoulos, S.E.; Lowik, C.W.G.M. Exposure of ks483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J. Bone Miner. Res. 2002, 17, 394–405. [Google Scholar] [CrossRef]
- Joyner, J.M.; Hutley, L.J.; Cameron, D.P. Estrogen receptors in human preadipocytes. Endocrine 2001, 15, 225–230. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Korach, K.S. Oestrogen receptor knockout mice: Roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003, 125, 143–149. [Google Scholar] [CrossRef]
- Wang, J.F.; Guo, Y.X.; Niu, J.Z.; Liu, J.; Wang, L.Q.; Li, P.H. Effects of radix puerariae flavones on liver lipid metabolism in ovariectomized rats. World J. Gastroenterol. 2004, 10, 1967–1970. [Google Scholar]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
- Shin, Y.W.; Bok, S.H.; Jeong, T.S.; Bae, K.H.; Jeoung, N.H.; Choi, M.S.; Lee, S.H.; Park, Y.B. Hypocholesterolemic effect of naringin associated with hepatic cholesterol regulating enzyme changes in rats. Int. J. Vitam. Nutr. Res. 1999, 69, 341–347. [Google Scholar] [CrossRef]
- Bok, S.H.; Lee, S.H.; Park, Y.B.; Bae, K.H.; Son, K.H.; Jeong, T.S.; Choi, M.S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-coa reductase and acyl coa: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Sample Availability: Samples of the C. unshiu peel extracts are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, D.W.; Lee, Y.; Kim, Y.T. Preventive Effects of Citrus unshiu Peel Extracts on Bone and Lipid Metabolism in OVX Rats. Molecules 2014, 19, 783-794. https://doi.org/10.3390/molecules19010783
Lim DW, Lee Y, Kim YT. Preventive Effects of Citrus unshiu Peel Extracts on Bone and Lipid Metabolism in OVX Rats. Molecules. 2014; 19(1):783-794. https://doi.org/10.3390/molecules19010783
Chicago/Turabian StyleLim, Dong Wook, Youngseok Lee, and Yun Tai Kim. 2014. "Preventive Effects of Citrus unshiu Peel Extracts on Bone and Lipid Metabolism in OVX Rats" Molecules 19, no. 1: 783-794. https://doi.org/10.3390/molecules19010783





