EPR Spectroscopy of a Clinically Active (1:2) Copper(II)-Histidine Complex Used in the Treatment of Menkes Disease: A Fourier Transform Analysis of a Fluid CW-EPR Spectrum
Abstract
:1. Introduction

2. Results and Discussion
2.1. EPR Theory


2.2. EPR Spectroscopy of (1:2)Copper(II)-l-Histidine

| Complex | g1 | g2 | g3 | A1(Cu) | A2(Cu) | A3(Cu) | A1(N) | A2(N) | A3(N) |
|---|---|---|---|---|---|---|---|---|---|
| [Cu(his)2] | 2.044 | 2.047 | 2.237 | 27 | 27 | 555 | 38/33 c | 38/33 c | 38/33 c |
 = 199 MHz. Nitrogen shf structure not satisfactorily resolved; c Simulation of low temperature EPR spectrum was performed using a mixture of four nitrogens (4N, splitting constant = 38 MHz) and three nitrogens (3NO, splitting constant = 33 MHz) assuming the ratio 4N:3NO = 0.8:0.2.
= 199 MHz. Nitrogen shf structure not satisfactorily resolved; c Simulation of low temperature EPR spectrum was performed using a mixture of four nitrogens (4N, splitting constant = 38 MHz) and three nitrogens (3NO, splitting constant = 33 MHz) assuming the ratio 4N:3NO = 0.8:0.2.2.3. Structural Characterization of Cu(L-His)2 Complex



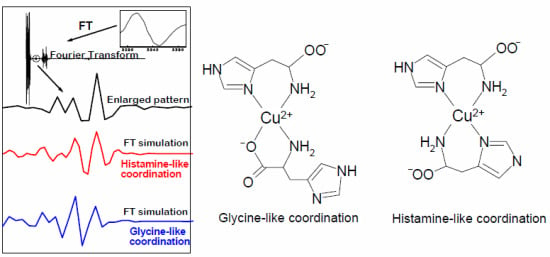
2.4. Fourier Transform of the CW-EPR Spectrum of Cu(L-His)2


3. Experimental
3.1. Metal-Chelate Solutions
3.2. EPR Measurements
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 8, 877–883. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants om normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Deschamps, P.; Kilkarni, P.P.; Gautam-Basak, M.; Sarkar, B. The saga of copper(II)-L-histidine. Coord. Chem. Rev. 2005, 249, 895–909. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef]
- Prasad, A.N.; Levin, S.; Rupar, C.A.; Prasad, C. Menkes disease and infantile epilepsy. Brain Dev. 2011, 33, 866–876. [Google Scholar] [CrossRef]
- Wilson, E.W.; Kasperian, M.H.; Martin, R.B. Binding of copper (II) to potentially tridentate amino acid ligands. J. Am. Chem. Soc. 1970, 92, 5365–5372. [Google Scholar] [CrossRef]
- Sigel, H.; McCormick, D.B. The structure of the copper(II)-L-histidine 1:2 complex in solution. J. Am. Chem. Soc. 1971, 93, 2041–2044. [Google Scholar] [CrossRef]
- Goodman, B.A.; McPhail, D.B.; Powell, H.K.J. Electron spin resonance study of copper(II)-amino acid complexes: Evidence for cis nad trans isomers and the structures of copper(II)-histidinate complexes in aqueous solution. J. Chem. Soc. Dalton Trans. 1981, 822–827. [Google Scholar] [CrossRef]
- Casella, L.; Gullotti, M. Coordination modes of histidine. 4. Coordination structures in the copper(II)-l-histidine (1:2) system. J. Inorg. Biochem. 1983, 18, 19–31. [Google Scholar] [CrossRef]
- Basosi, R.; Valensin, G.; Gaggelli, E.; Froncisz, W.; Pasenkiewicz-Gierula, M.; Antholine, W.E.; Hyde, J.S. Multifrequency ESR of Cu(II)-(His)n (His = Histidine). 1. Immobile phase. Inorg. Chem. 1986, 25, 3006–3010. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Froncisz, W.; Basosi, R.; Antholine, W.E.; Hyde, J.S. Multifrequency ESR with fourier analysis of CuII(His)n (His = Histidine) 2. Mobile phase. Inorg. Chem. 1987, 26, 801–805. [Google Scholar] [CrossRef]
- Romanelli, M.; Basosi, R. An electron spin-echo study of Cu(II)-(His)n in frozen aqueous solution. Chem. Phys. Lett. 1988, 143, 404–408. [Google Scholar] [CrossRef]
- Colaneri, M.; Peisach, J. An electron spin-echo envelope modulation study of copper(II)-doped single crystals of L-histidine hydrochloride monohydrate. J. Am. Chem. Soc. 1992, 114, 5335–5341. [Google Scholar] [CrossRef]
- Manikandan, P.; Epel, B.; Goldfarb, D. Structure of copper(II)-histidine based complexes in frozen aqueous solutions as determined from high-field pulsed electron nuclear double resonance. Inorg. Chem. 2001, 40, 781–787. [Google Scholar] [CrossRef]
- Deschamps, P.; Prasad, P.K.; Sarkar, B. X-ray structure of physiological Copper(II)-Bis(l-histidinato) complex. Inorg. Chem. 2004, 43, 3338–3340. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Mazur, M.; Telser, J.; McInnes, E.J.L.; Mabbs, F.E. High-Affinity Binding Site for Copper(II) in human and dog serum albumins (an EPR study). J. Phys. Chem. B 1999, 103, 5591–5597. [Google Scholar] [CrossRef]
- Husarikova, L.; Repicka, Z.; Moncol, J.; Valigura, D.; Valko, M.; Mazur, M. Unusual EPR spectra with inverse axial g values of Chlorosalicylate-Cu(II)-2,6-Pyridinedimethanol complex in frozen water-methanol solution. Appl. Magn. Reson. 2013, 44, 571–582. [Google Scholar] [CrossRef]
- Dela Lunga, G.; Pogni, R.; Basosi, R. Discrimination of copper-nitrogen ligand coordination by Fourier-analysis of EPR spectra in mobile phase. J. Magn. Reson. A 1995, 114, 174–178. [Google Scholar] [CrossRef]
- Dunham, W.R.; Fee, J.A.; Harding, L.J.; Grande, H.J. Application of fast fourier transforms to EPR spectra of free radicals in solution. J. Magn. Reson. 1980, 40, 351–359. [Google Scholar]
- Mabbs, F.E.; Collison, D. Electron Paramagnetic Resonance of d Transition Metal Compounds; Elsevier: Amsterdam, The Netherlands, 1992; p. 20. [Google Scholar]
- Froncisz, W.; Hyde, J.S. Broadening by strains of lines in the g-Parallel region of Cu2+ EPR spectra. J. Chem. Phys. 1980, 73, 3123–3131. [Google Scholar] [CrossRef]
- Liczwek, D.L.; Belford, R.L.; Pilbrow, J.R.; Hyde, J.S. Elevation of copper nuclear quadrupole coupling in thio complexes by completion of the coordination sphere. J. Phys. Chem. 1983, 87, 2509–2512. [Google Scholar] [CrossRef]
- Seebauer, E.G.; Duliba, E.P.; Scogin, D.A.; Gennis, R.B.; Belford, R.L. EPR evidence on the structure of the copper(II)-bacitracin A complex. J. Am. Chem. Soc. 1983, 105, 4926–4929. [Google Scholar] [CrossRef]
- Hyde, J.S.; Froncisz, W. The role of microwave frequency in EPR spectroscopy of copper complexes. Ann. Rev. Biophys. Bioeng. 1982, 11, 391–417. [Google Scholar] [CrossRef]
- Edgcomb, S.P.; Murphy, K.P. Variability in the pKa of histidine side-chains correlates with burial within proteins. Proteins 2002, 49, 1–6. [Google Scholar] [CrossRef]
- Szabo-Planka, T.; Peintler, G.; Rockenabuer, A.; Gyor, M.; Vargafabian, M.; Institorisz, L.; Balazspiri, L. Electron Spin Resonance study of copper(II) complexes of x-glycine and glycyl-x type dipeptides and related tripeptides—variation of coordination modes with ligand excess and pH in fluid and frozen aqueous-solutions. J. Chem. Soc. Dalton Trans. 1989, 1925–1932. [Google Scholar]
- Szabo-Planka, T.; Rockenbauer, A.; Korecz, L. ESR study of the copper(II)-glycylglycine equilibrium system in fluid aqueous solution. Computer analysis of overlapping multispecies spectra. Magn. Reson. Chem. 1999, 37, 484–492. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]
- Evertsson, B. The crystal structure of bis-l-histidinecopper(II) dinitrate dehydrate. Acta Cryst. 1969, B25, 30–41. [Google Scholar] [CrossRef]
- Colaneri, M.K.; Peisach, J. A single-crystal EPR and ESEEM analysis of Cu(II)-Doped Bis(L-Histidinato)Cadmium dihydrate. J. Am. Chem. Soc. 1995, 117, 6308–6315. [Google Scholar] [CrossRef]
- Laurie, S.H. Handbook of Metal-Ligand Interactions in Biological Fluids—Bioinorganic Chemistry; Berthon, G., Ed.; Marcel Dekker: New York, NY, USA, 1995; Volume 2, p. 603. [Google Scholar]
- Belford, R.L.; Nilges, M.J. Computer Simulation of Powder Spectra, EPR Symposium. In Proceedings of the 21st Rocky Mountain Conference, Denver, CO, USA, 11–15 August 1979.
- Nilges, M.J. Electron Paramagnetic Resonance Studies of Low Symmetry Nickel(I) and Molybdenum(V) Complexes. Ph.D. Thesis, University of Illinois, Urbana, IL, USA, 1979. [Google Scholar]
- Pelikan, P.; Liska, M.; Valko, M.; Mazur, M. Quantitative analysis of EPR spectra of powdered samples containing a mixture of various paramagnetic particles. J. Magn. Reson. A 1996, 122, 9–15. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gala, L.; Lawson, M.; Jomova, K.; Zelenicky, L.; Congradyova, A.; Mazur, M.; Valko, M. EPR Spectroscopy of a Clinically Active (1:2) Copper(II)-Histidine Complex Used in the Treatment of Menkes Disease: A Fourier Transform Analysis of a Fluid CW-EPR Spectrum. Molecules 2014, 19, 980-991. https://doi.org/10.3390/molecules19010980
Gala L, Lawson M, Jomova K, Zelenicky L, Congradyova A, Mazur M, Valko M. EPR Spectroscopy of a Clinically Active (1:2) Copper(II)-Histidine Complex Used in the Treatment of Menkes Disease: A Fourier Transform Analysis of a Fluid CW-EPR Spectrum. Molecules. 2014; 19(1):980-991. https://doi.org/10.3390/molecules19010980
Chicago/Turabian StyleGala, Lukas, Michael Lawson, Klaudia Jomova, Lubomir Zelenicky, Andrea Congradyova, Milan Mazur, and Marian Valko. 2014. "EPR Spectroscopy of a Clinically Active (1:2) Copper(II)-Histidine Complex Used in the Treatment of Menkes Disease: A Fourier Transform Analysis of a Fluid CW-EPR Spectrum" Molecules 19, no. 1: 980-991. https://doi.org/10.3390/molecules19010980