Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains
Abstract
:1. Introduction
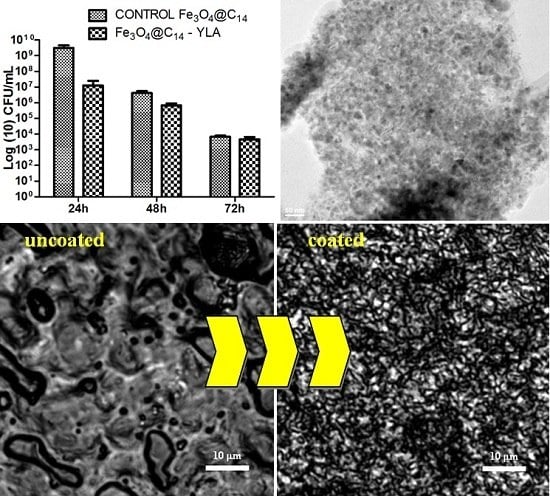
2. Results and Discussion







3. Experimental Section
3.1. Preparation of Iron Oxide@C14 Nanoparticles
3.2. Surface Modification of Catheter Pieces with Essential Oils Stabilized by Iron Oxide@C14 Nanoparticles
3.3. Characterization
3.3.1. TEM
3.3.2. XRD
3.3.3. TGA
3.4. Microbial Biofilms Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 2001, 17, 280–285. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Waomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar]
- Deans, S.G.; Ritchie, G. Antibacterial Properties of Plant Essential Oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar]
- George, D.R.; Smith, T.J.; Shiel, R.S.; Sparagano, O.A.E.; Guy, J.H. Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2009, 161, 276–282. [Google Scholar]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E-coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar]
- Tserennadmid, R.; Tako, M.; Galgoczy, L.; Papp, T.; Pesti, M.; Vagvolgyi, C.; Almassy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Kavanaugh, N.L.; Ribbeck, K. Selected Antimicrobial Essential Oils Eradicate Pseudomonas spp. and Staphylococcus aureus Biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar]
- Hussin, N.; Mondello, L.; Costa, R.; Dugo, P.; Yusoff, N.I.N.; Yarmo, M.A.; Ab Wahab, A.; Said, M. Quantitative and Physical Evaluation of Patchouli Essential Oils Obtained from Different Sources of Pogostemon cablin. Nat. Prod. Commun. 2012, 7, 927–930. [Google Scholar]
- Yang, X.; Zhang, X.; Yang, S.P.; Liu, W.Q. Evaluation of the Antibacterial Activity of Patchouli Oil. Iran. J. Pharm. Res. 2013, 12, 307–316. [Google Scholar]
- Takahashi, M.; Inai, Y.; Miyazawa, N.; Kurobayashi, Y.; Fujita, A. Identification of the Key Odorants in Tahitian Cured Vanilla Beans (Vanilla tahitensis) by GC-MS and an Aroma Extract Dilution Analysis. Biosci. Biotechnol. Biochem. 2013, 77, 601–605. [Google Scholar]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar]
- Subramanian, S.; Banu, H.H.; Bai, R.M.R.; Shanmugavalli, R. Biochemical evaluation of antihyperglycemic and antioxidant nature of Psidium guajava leaves extract in streptozotocin-induced experimental diabetes in rats. Pharm. Biol. 2009, 47, 298–303. [Google Scholar]
- Brokl, M.; Fauconnier, M.L.; Benini, C.; Lognay, G.; du Jardin, P.; Focant, J.F. Improvement of Ylang-Ylang Essential Oil Characterization by GCxGC-TOFMS. Molecules 2013, 18, 1783–1797. [Google Scholar]
- Tadtong, S.; Suppawat, S.; Tintawee, A.; Saramas, P.; Jareonvong, S.; Hongratanaworakit, T. Antimicrobial Activity of Blended Essential Oil Preparation. Nat. Prod. Commun. 2012, 7, 1401–1404. [Google Scholar]
- Lee, K.; Lim, E.J.; Kim, K.S.; Huang, S.L.; Veeranagouda, Y.; Rehm, B.H.A. An alginate-like exopolysaccharide biosynthesis gene cluster involved in biofilm aerial structure formation by Pseudomonas alkylphenolia. Appl. Microbiol. Biotechnol. 2014, 98, 4137–4148. [Google Scholar]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazar, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.; Lazar, V. Inhibitory Activity of Fe3O4/Oleic Acid/Usnic Acid-Core/Shell/Extra-Shell Nanofluid on S. aureus Biofilm Development. IEEE Trans. Nanobiosci. 2011, 10, 269–274. [Google Scholar]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.M.J.; Huang, C.M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar]
- Schleich, N.; Sibret, P.; Danhier, P.; Ucakar, B.; Laurent, S.; Muller, R.N.; Jerome, C.; Gallez, B.; Preat, V.; Danhier, F. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int. J. Pharm. 2013, 447, 94–101. [Google Scholar]
- Philosof-Mazor, L.; Dakwar, G.R.; Popov, M.; Kolusheva, S.; Shames, A.; Linder, C.; Greenberg, S.; Heldman, E.; Stepensky, D.; Jelinek, R. Bolaamphiphilic vesicles encapsulating iron oxide nanoparticles: New vehicles for magnetically targeted drug delivery. Int. J. Pharm. 2013, 450, 241–249. [Google Scholar]
- Wang, C.H.; Qiao, L.; Zhang, Q.; Yan, H.S.; Liu, K.L. Enhanced cell uptake of superparamagnetic iron oxide nanoparticles through direct chemisorption of FITC-Tat-PEG(600)-b-poly(glycerol monoacrylate). Int. J. Pharm. 2012, 430, 372–380. [Google Scholar]
- Meenach, S.A.; Otu, C.G.; Anderson, K.W.; Hilt, J.Z. Controlled synergistic delivery of paclitaxel and heat from poly(beta-amino ester)/iron oxide-based hydrogel nanocomposites. Int. J. Pharm. 2012, 427, 177–184. [Google Scholar]
- Masoudi, A.; Hosseini, H.R.M.; Shokrgozar, M.A.; Ahmadi, R.; Oghabian, M.A. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm. 2012, 433, 129–141. [Google Scholar]
- Lee, E.S.; Lim, C.; Song, H.T.; Yun, J.M.; Lee, K.S.; Lee, B.J.; Youn, Y.S.; Oh, Y.T.; Oh, K.T. A nanosized delivery system of superparamagnetic iron oxide for tumor MR imaging. Int. J. Pharm. 2012, 439, 342–348. [Google Scholar]
- Jansch, M.; Stumpf, P.; Graf, C.; Ruhl, E.; Muller, R.H. Adsorption kinetics of plasma proteins on ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles. Int. J. Pharm. 2012, 428, 125–133. [Google Scholar]
- Chen, X.L.; Lv, H.Y.; Ye, M.; Wang, S.Y.; Ni, E.R.; Zeng, F.W.; Cao, C.; Luo, F.H.; Yan, J.H. Novel superparamagnetic iron oxide nanoparticles for tumor embolization application: Preparation, characterization and double targeting. Int. J. Pharm. 2012, 426, 248–255. [Google Scholar]
- Mihaiescu, D.E.; Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Ficai, A.; Vasile, O.R.; Bleotu, C.; Saviuc, C. Prosthetic Devices with Functionalized Anti-biofilm Surface Based NanoAg@C18. Curr. Org. Chem. 2013, 17, 105–112. [Google Scholar]
- Mihaiescu, D.E.; Cristescu, R.; Dorcioman, G.; Popescu, C.E.; Nita, C.; Socol, G.; Mihailescu, I.N.; Grumezescu, A.M.; Tamas, D.; Enculescu, M.; et al. Functionalized magnetite silica thin films fabricated by MAPLE with antibiofilm properties. Biofabrication 2013, 5. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Ficai, A.; Chifiriuc, C.M.; Lazar, V.; Radulescu, R. Fe3O4@C-18-carvone to prevent Candida tropicalis biofilm development. Rev. Rom. Mater. 2013, 43, 300–305. [Google Scholar]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, C.M.; Radulescu, R. Magnetite—Usnic Acid Nanostructured Bioactive Material with Antimicrobial Activity. Rev. Rom. Mater. 2013, 43, 402–407. [Google Scholar]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. 2008, 322, 207–228. [Google Scholar]
- Magesh, H.; Kumar, A.; Alam, A.; Priyam; Sekar, U.; Sumantran, V.N.; Vaidyanathan, R. Identification of natural compounds which inhibit biofilm formation in clinical isolates of Klebsiella pneumoniae. Indian J. Exp. Biol. 2013, 51, 764–772. [Google Scholar]
- Coenye, T.; Brackman, G.; Rigole, P.; de Witte, E.; Honraet, K.; Rossel, B.; Nelis, H.J. Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine 2012, 19, 409–412. [Google Scholar]
- Ravichandiran, V.; Shanmugam, K.; Anupama, K.; Thomas, S.; Princy, A. Structure-based virtual screening for plant-derived SdiA-selective ligands as potential antivirulent agents against uropathogenic Escherichia coli. Eur. J. Med. Chem. 2012, 48, 200–205. [Google Scholar]
- Abraham, S.V.P.I.; Palani, A.; Ramaswamy, B.R.; Shunmugiah, K.P.; Arumugam, V.R. Antiquorum Sensing and Antibiofilm Potential of Capparis spinosa. Arch. Med. Res. 2011, 42, 658–668. [Google Scholar]
- Singh, B.N.; Singh, H.B.; Singh, A.; Singh, B.R.; Mishra, A.; Nautiyal, C.S. Lagerstroemia speciosa fruit extract modulates quorum sensing-controlled virulence factor production and biofilm formation in Pseudomonas aeruginosa. Microbiology 2012, 158, 529–538. [Google Scholar]
- Harjai, K.; Kumar, R.; Singh, S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2010, 58, 161–168. [Google Scholar]
- Trentin, D.D.; Giordani, R.B.; Zimmer, K.R.; da Silva, A.G.; da Silva, M.V.; Correia, M.T.D.; Baumvol, I.J.R.; Macedo, A.J. Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar]
- Carneiro, V.A.; dos Santos, H.S.; Arruda, F.V.S.; Bandeira, P.N.; Albuquerque, M.R.J.R.; Pereira, M.O.; Henriques, M.; Cavada, B.S.; Teixeira, E.H. Casbane Diterpene as a Promising Natural Antimicrobial Agent against Biofilm-Associated Infections. Molecules 2011, 16, 190–201. [Google Scholar]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics in Vitro and in Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [Green Version]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar]
- Anghel, I.; Holban, A.M.; Andronescu, E.; Grumezescu, A.M.; Chifiriuc, M.C. Efficient surface functionalization of wound dressings by a phytoactive nanocoating refractory to Candida albicans biofilm development. Biointerphases 2013, 8. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar]
- Vázquez-Sánchez, D.; Cabo, M.L.; Rodríguez-Herrera, J.J. Antimicrobial Activity of Essential Oils against Staphylococcus Aureus Biofilms. Food Sci. Technol. Int. 2014. in press. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25280938 (accessed on 28 October 2014).
- Wu, H.Q.; Li, L.; Li, J.; He, Z.D.; Liu, Z.G.; Zeng, Q.Q.; Wang, Y.S. Acaricidal Activity of DHEMH, Derived from Patchouli Oil, against House Dust Mite, Dermatophagoides farinae. Chem. Pharm. Bull. 2012, 60, 178–182. [Google Scholar]
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar]
- Shin, S. Anti-Aspergillus activities of plant essential oils and their combination effects with ketoconazole or amphotericin B. Arch. Pharm. Res. 2003, 26, 389–393. [Google Scholar]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar]
- U.S. Pharmacopeia. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c51 (accessed on 2 November 2014).
- Voicu, G.; Andronescu, E.; Grumezescu, A.M.; Huang, K.S.; Ficai, A.; Yang, C.H.; Bleotu, C.; Chifiriuc, M.C. Antitumor Activity of Magnetite Nanoparticles: Influence of Hydrocarbonated Chain of Saturated Aliphatic Monocarboxylic Acids. Curr. Org. Chem. 2013, 17, 831–840. [Google Scholar]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.E.; Stanciu, G.A.; Andronescu, E. Hybrid Nanomaterial for Stabilizing the Antibiofilm Activity of Eugenia carryophyllata Essential Oil. IEEE Trans. Nanobiosci. 2012, 11, 360–365. [Google Scholar]
- Sample Availability: Samples of the nanostructures are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoșanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules 2014, 19, 17943-17956. https://doi.org/10.3390/molecules191117943
Bilcu M, Grumezescu AM, Oprea AE, Popescu RC, Mogoșanu GD, Hristu R, Stanciu GA, Mihailescu DF, Lazar V, Bezirtzoglou E, et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules. 2014; 19(11):17943-17956. https://doi.org/10.3390/molecules191117943
Chicago/Turabian StyleBilcu, Maxim, Alexandru Mihai Grumezescu, Alexandra Elena Oprea, Roxana Cristina Popescu, George Dan Mogoșanu, Radu Hristu, George A. Stanciu, Dan Florin Mihailescu, Veronica Lazar, Eugenia Bezirtzoglou, and et al. 2014. "Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains" Molecules 19, no. 11: 17943-17956. https://doi.org/10.3390/molecules191117943