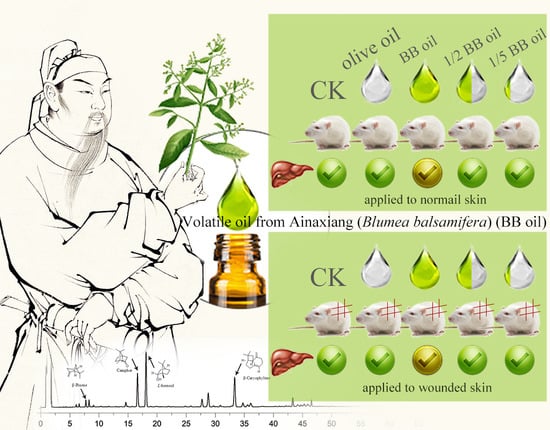
External Application of the Volatile Oil from Blumea balsamifera May Be Safe for Liver — A Study on Its Chemical Composition and Hepatotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Phytochemical Analysis
| No. | RI a | Constituent b | Peak Area (%) c |
|---|---|---|---|
| 1 | 814 | (E)-2-Hexenal | 0.04 |
| 2 | 860 | 1-Hexanol | 0.21 |
| 3 | 868 | (3 E)-3-Hexen-l-ol | 0.11 |
| 4 | 868 | (E)-2-Hexen-l-ol | 0.08 |
| 5 | 874 | n-Butyl acrylate | 0.26 |
| 6 | 943 | β-Pinene | 5.2 |
| 7 | 943 | Camphene | 1.5 |
| 8 | 948 | α-Pinene | 2.04 |
| 9 | 952 | 3-Octanone | 0.21 |
| 10 | 958 | β-Myrcene | 0.1 |
| 11 | 969 | 1-Octen-3-ol | 8.31 |
| 12 | 976 | (E)-β-Ocimene | 0.19 |
| 13 | 976 | (Z)-β-Ocimene | 0.76 |
| 14 | 979 | 3-Octanol | 1.36 |
| 15 | 1018 | (+)-Limonene | 0.39 |
| 16 | 1042 | o-Cymene | 0.24 |
| 17 | 1059 | 1,8-Cineole | 0.06 |
| 18 | 1072 | Hotrienol | 0.28 |
| 19 | 1082 | Linalool | 1.76 |
| 20 | 1119 | Chrysanthenone | 0.22 |
| 21 | 1121 | D-Camphor | 9.54 |
| 22 | 1137 | Terpinenol-4 | 0.09 |
| 23 | 1138 | l-Borneol | 43.55 |
| 24 | 1143 | α-Terpinenol | 0.24 |
| 25 | 1207 | (−)-Perillaaldehyde | 0.13 |
| 26 | 1230 | Cuminaldehyde | 0.1 |
| 27 | 1277 | Bornyl acetate | 0.41 |
| 28 | 1351 | 1,3,4,5,6,7-Hexahydro-2,5,5-trimethyl-2H-2,4a-ethanonaphthalene | 2.93 |
| 29 | 1386 | 2-tert-Butyl-1,4-dimethoxybenzene | 3.07 |
| 30 | 1387 | Longifolene-(V4) | 2.14 |
| 31 | 1396 | Dehydroaromadendrene | 0.11 |
| 32 | 1419 | (−)-α-Gurjunene | 0.22 |
| 33 | 1435 | (−)-γ-Cadinene | 0.2 |
| 34 | 1462 | Alloaromadendrene oxide-(1) | 0.34 |
| 35 | 1494 | (E)-β-Caryophyllene | 6.51 |
| 36 | 1507 | Caryophyllene oxide | 0.89 |
| 37 | 1530 | Ledol | 0.12 |
| 38 | 1579 | β-Caryophyllene | 1.54 |
| 39 | 1614 | Guaiol | 0.41 |
| 40 | 1626 | γ-Eudesmol | 0.33 |
| 41 | 1628 | Xanthoxyline | 1.4 |
| Sum | 97.59 |
2.1.2. Effect of Different Concentrations of BB Oil on Plasma ALT Levels in Normal and Wounded Rats

2.1.3. Effect of Different Concentrations of BB Oil on Plasma AST Levels in Normal and Wound Bearing Rats

2.1.4. Effect of Different Concentrations of BB Oil on Plasma ALP Levels in Normal and Wound Bearing Rats
2.1.5. Effect of Different Concentrations of BB Oil on Plasma Total Bilirubin Levels in Normal and Wound Bearing Rats


2.1.6. Histopathology of Liver Samples after Treatment with Different Concentrations of BB Oil in Normal Rats
2.1.7. Histopathology of Liver Samples after Treatment with Different Concentrations of BB Oil in Wound Bearing Rats
2.2. Discussion


3. Experimental Section
3.1. Plant Material
3.2. Volatile Oil Obtaining
3.3. GC-FID Analysis
3.4. GC-MS Analysis
3.5. Identification of Constituents of Essential Oils
3.6. Experimental Animals
3.7. Animal Model
| Group No. | Treatments | Doses (mg/kg) |
|---|---|---|
| A1 | Control | 0 |
| A2 | Olive oil (vehicle) | 1000 |
| A3 | BB oil (100% w/v) | 1000 |
| A4 | BB oil (50% w/v) | 1000 |
| A5 | BB oil (20% w/v) | 1000 |
| Group No. | Treatments | Doses (mg/kg) |
|---|---|---|
| B1 | Control | 0 |
| B2 | Olive oil (vehicle) | 1000 |
| B3 | BB oil (100% w/v) | 1000 |
| B4 | BB oil (50% w/v) | 1000 |
| B5 | BB oil (20% w/v) | 1000 |
3.8. Sample Collection
3.9. Clinical Chemistry
3.10. Histopathologic Evaluation
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiangsu New Medical College. Dictionary of Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1977. [Google Scholar]
- Guizhou Institute of Traditional Chinese Medicine. Guizhou Chinese Medicine Resources; Chinese Medical Science Press: Beijing, China, 1992; p. 262. [Google Scholar]
- Zhari, I.; Norhayati, I.; Jaafar, L. Malaysian Herbal Monograph; Malaysia Monograph Committee: Kuala Lumpur, Malaysia, 1999; Volume 1, pp. 9–12. [Google Scholar]
- Ragasa, C.Y.; Kristin C. Co., A.L.; Rideout, J.A. Antifungal metabolites from Blumea balsamifera. Nat. Prod. Res. 2005, 19, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.Z.; Chen, H.H.; Wang, H.B.; Qin, S.; Zhu, W.Y.; Xu, L.H.; Jiang, C.L.; Li, W.J. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett. Appl. Microbiol. 2008, 47, 574–580. [Google Scholar]
- Sakee, U.; Maneerat, S.; Cushnie, T.P.; De-eknamkul, W. Antimicrobial activity of Blumea balsamifera (Lin.) DC. extracts and essential oil. Nat. Prod. Res. 2011, 25, 1849–1856. [Google Scholar]
- Nessa, F.; Ismail, Z.; Mohamed, N.; Haris, M.R.H.M. Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem. 2004, 88, 243–252. [Google Scholar] [CrossRef]
- Fazilatun, N.; Nornisah, M.; Zhari, I. Superoxide radical scavenging properties of extracts and flavonoids isolated from the leaves of Blumea balsamifera. Pharm. Bio. 2005, 43, 15–20. [Google Scholar] [CrossRef]
- Osaki, N.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Sesquiterpenoids and plasmin-inhibitory flavonoids from Blumea balsamifera. J. Nat. Prod. 2005, 68, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Kojima-Yuasa, A.; Morii, R.; Huang, X.D.; Norikura, T.; Rho, S.N.; Matsui-Yuasa, I. Anti-obesity effect of Blumea balsamifera extract in 3T3-L1 preadipocytes and adipocytes. Am. J. Chin. Med. 2009, 37, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Yamada, Y.; Komiyama, K.; Hayashi, M.; Ishibashi, M.; Yoshida, T.; Koyano, T.; Kam, T.S.; Murata, K.; Sugahara, K.; et al. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood 2006, 107, 679–688. [Google Scholar]
- Norikura, T.; Kojima-Yuasa, A.; Shimizu, M.; Huang, X.D.; Xu, S.H.; Kametani, S.; Rho, S.N.; Kennedy, D.O.; Matsui-Yuasa, I. Anticancer activities and mechanisms of Blumea balsamifera extract in hepatocellular carcinoma Cells. Am. J. Chin. Med. 2008, 36, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Mounika, C.P.; Kumar, K.A.; Soumya, M.; Rajaram, C.; Kumar, S.N. Antidiarrheal activity of ethanolic extract of Blumea balsamifera in experimental animal models. Int. J. Nov. Asp. Pharm. Res. 2013, 1, 14–19. [Google Scholar]
- Ruangrungsi, N.; Tappayuthpijarn, P.; Tantivatana, P.; Borris, R.P.; Cordell, G.A. Traditional medicinal plants of Thailand. I. isolation and structure elucidation of two new flavonoids, (2R,3R)-dihydroquercetin-4′-methyl ether and (2R,3R)-dihydroquercetin-4′,7-dimethyl ether from Blumea balsamifera. J. Nat. Prod. 1981, 44, 541–545. [Google Scholar]
- Ali, D.M.H.; Wong, K.C.; Lim, P.K. Flavonoids from Blumea balsamifera. Fitoterapia 2005, 76, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Kang, H.; Yao, G.H.; Zeng, W.Z. Study on the chemical constituents of Blumea balsamifera. Chin. Tradit. Herb. Drugs 2007, 38, 350–352. (In Chinese) [Google Scholar]
- Yan, Q.X.; Tan, D.P.; Kang, H.; Feng, H.L.; Zeng, W.Z. Study on flavonoids constituents of Blumea balsamifera. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 86–89. (In Chinese) [Google Scholar]
- Shirota, O.; Oribello, J.M.; Sekita, S.; Satake, M. Sesquiterpenes from Blumea balsamifera. J. Nat. Prod. 2011, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, X.S.; Zhao, C. Chemical components of volatile oil from Folium et Cacumen Blumeae balsamiferae originated from Guizhou. J. Instrum. Anal. 2001, 20, 76–78. (In Chinese) [Google Scholar]
- Bhuiyan, M.N.I.; Jasim, U.C.; Jaripa, B. Chemical components in volatile oil from Blumea balsamifera (L.) DC. Bangladesh. J. Bot. 2009, 38, 107–109. [Google Scholar]
- Xu, S.B.; Zhao, J.H. Protective actions of Blumea flavanones on experimental liver injury. Chin. Pharm. Bull. 1998, 14, 191–192. (In Chinese) [Google Scholar]
- Pu, H.L.; Zhao, J.H.; Xu, S.B.; Hu, Q. Protective actions of Blumea flavanones on primary cultured hepatocytes against lipid peroxidation. Chin. Tradit. Herb. Drugs 2000, 31, 113–115. (In Chinese) [Google Scholar]
- Norikura, T.; Kojima-Yuasa, A.; Shimizu, M.; Huang, X.D.; Xu, S.H.; Kametani, S.; Rho, S.N.; Kennedy, D.O.; Matsui-Yuasa, I. Mechanism of growth inhibitory effect of Blumea balsamifera extract in hepatocellular carcinoma. Biosci. Biotechnol. Biochem. 2008, 72, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Yu, H.J.; Pan, A.; Du, J.Y.; Ruan, Y.C.; Ko, W.H.; Chan, H.C.; Zhou, W.L. Cellular mechanisms underlying the laxative effect of flavonol naringenin on rat constipation model. PLoS One 2008, 3, e3348. [Google Scholar] [CrossRef]
- Baggio, C.H.; Freitas, C.S.; Mayer, B.; Dos Santos, A.C.; Twardowschy, A.; Potrich, F.B.; Cipriani, T.R.; de Souza, L.M.; Sassaki, G.L.; Iacomini, M. Muscarinic-dependent inhibition of gastric emptying and intestinal motility by fractions of Maytenus ilicifolia Mart ex. Reissek. J. Ethnopharmacol. 2009, 123, 385–391. [Google Scholar] [CrossRef]
- Saleh, I.G.; Ali, Z.; Abe, N.; Wilson, F.D.; Hamada, F.M.; Abd-Ellah, M.F.; Walker, L.A.; Khan, I.A.; Ashfaq, M.K. Effect of green tea and its polyphenols on mouse liver. Fitoterapia 2013, 90, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.B.; Rumack, B.H.; Thomas, H.; Penterson, R.G.; Bryson, P. Pennyroyal oil poisoning and hepatotoxicity. J. Am. Med. Assoc. 1979, 242, 2873–2874. [Google Scholar] [CrossRef]
- Pandey, R. Studies on phytonematotoxic properties in the extract of some medicinal and aromatic plants. Int. Nematol. Netw. Newsl. 1990, 7, 19–20. [Google Scholar]
- Teng, Y.W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.G.; Nehra, V.; Lindor, K.D.; Buchman, A.L. Metabolic and nutritional considerations in nonalcoholic fatty liver. Hepatology 2000, 32, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fu, W.J.; Pang, Y.X.; Wang, H.; Hu, X.; Nie, H. The study of skin allergy and acute toxicity of Blumea balsamifera oil. Chin. J. Trop. Crops 2013, 12, 2499–4502. (In Chinese) [Google Scholar]
- Fishel, R.; Barbul, A.; Wasserkrug, H.L.; Penberthy, L.T.; Rettura, G.; Efron, G. Cyclosporine a impairs wound healing in rats. J. Surg. Res. 1983, 34, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K. The Methodology of Pharmacological Experiments of Traditional Chinese Medicine; Shanghai Science and Technology Press: Shanghai, China, 2006. [Google Scholar]
- Chen, Q. Methodology on Chinese Medicinal Pharmacology; People’s Medical Publishing Press: Beijing, China, 2006. [Google Scholar]
- Matsumoto, A.; Sakurai, S.; Shinriki, N.; Suzuki, S.; Miura, T. Therapeutic effects of ozonized Olive oil in the treatment of intractable fistula and wound after surgical operation. In Proceedings of the 15th World Ozone Congress, London, UK, 11–15 September 2001; Volume 11, No. 15. pp. 77–84.
- Purba, M.; Kouris-Blazos, A.; Wattanapenpaiboon, N.; Lukito, W.; Rothenberg, E.M.; Steen, B.C.; Wahlqvist, M.L. Skin wrinkling: Can food make a difference? J. Am. Coll. Nutr. 2001, 20, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: Partially controlled, single-blinded study. Complement. Ther. Med. 2003, 11, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.P.; Mc Ginty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on l-borneol. Food Chem. Toxicol. 2008, 46, S81–S84. [Google Scholar] [PubMed]
- Fu, W.J.; Wang, D.; Pang, Y.X.; Wang, H.; Wang, Z.; Nie, H.; Yu, F.L.; Zhang, Y.B. Effect of Blumea balsamifera oil on percutaneous absorption of salbutamol sulfate. Chin. J. Exp. Tradit. Med. Form. 2013, 19, 174–177. (In Chinese) [Google Scholar]
- Pang, Y.X.; Wang, D.; Fan, Z.W.; Chen, X.L.; Yu, F.L.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera—A phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds D-Camphor and β-pinene are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.-X.; Fan, Z.-W.; Wang, D.; Yang, Q.; Wang, K.; Chen, X.-L.; Hu, X.; Yu, F.-L.; Chen, Z.-X. External Application of the Volatile Oil from Blumea balsamifera May Be Safe for Liver — A Study on Its Chemical Composition and Hepatotoxicity. Molecules 2014, 19, 18479-18492. https://doi.org/10.3390/molecules191118479
Pang Y-X, Fan Z-W, Wang D, Yang Q, Wang K, Chen X-L, Hu X, Yu F-L, Chen Z-X. External Application of the Volatile Oil from Blumea balsamifera May Be Safe for Liver — A Study on Its Chemical Composition and Hepatotoxicity. Molecules. 2014; 19(11):18479-18492. https://doi.org/10.3390/molecules191118479
Chicago/Turabian StylePang, Yu-Xin, Zuo-Wang Fan, Dan Wang, Quan Yang, Kai Wang, Xiao-Lu Chen, Xuan Hu, Fu-Lai Yu, and Zhen-Xia Chen. 2014. "External Application of the Volatile Oil from Blumea balsamifera May Be Safe for Liver — A Study on Its Chemical Composition and Hepatotoxicity" Molecules 19, no. 11: 18479-18492. https://doi.org/10.3390/molecules191118479