Mechanisms Underlying Vasorelaxation Induced in Rat Aorta by Galetin 3,6-Dimethyl Ether, a Flavonoid from Piptadenia stipulacea (Benth.) Ducke
Abstract
:1. Introduction

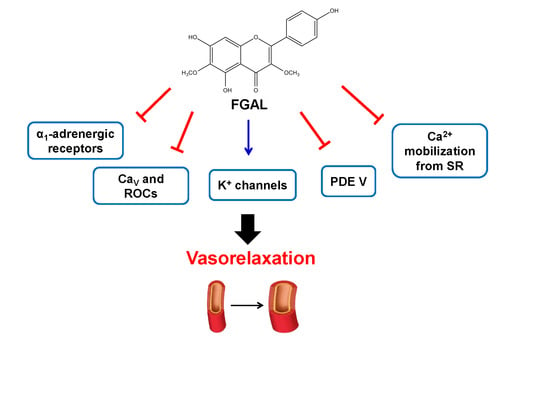
2. Results and Discussion


| [FGAL] M | Emax (%) | pD2 |
|---|---|---|
| Control | 100.0 ± 0.0 | 8.13 ± 0.18 |
| 3 × 10−6 | 94.9 ± 2.7 | 7.75 ± 0.10 |
| 10−5 | 60.9 ± 8.2 *** ## | 7.49 ± 0.16 |
| 3 × 10−5 | 3.5 ± 2.3 *** ¥¥¥ | Nd |


| Compounds | Emax (%) | pD2 |
|---|---|---|
| FGAL | 100.0 ± 0.0 | 5.35 ± 0.11 |
| 10 mM TEA+ + FGAL | 98.7 ± 1.3 | 4.71 ± 0.06 ** |
| 1 mM TEA+ + FGAL | 92.8 ± 3.4 | 5.57 ± 0.17 |
| 10−5 M glibenclamide + FGAL | 98.8 ± 1.2 | 4.79 ± 0.05 * |
| 5 × 10−8 M apamin + FGAL | 98.3 ± 1.1 | 4.75 ± 0.11 * |
| 10−4 M BaCl2 + FGAL | 89.1 ± 4.6 * | 4.62 ± 0.17 ** |
| 10−3 M 4-AP + FGAL | 86.2 ± 3.7 ** | 4.82 ± 0.11 * |
| [FGAL] M | Emax (%) | pD2 |
|---|---|---|
| Control | 100.0 ± 0.0 | 2.52 ± 0.10 |
| 10−5 | 94.3 ± 2.7 | 1.95 ± 0.12 ** |
| 3 × 10−5 | 61.7 ± 8.1 *** ### | 2.69 ± 0.08 |
| 10−4 | 9.7 ± 1.9 *** ¥¥¥ | Nd |
| 3 × 10−4 | 1.1 ± 0.8 *** | Nd |

| [FGAL] M | Emax (%) | pD2 |
|---|---|---|
| Control | 100.0 ± 0.0 | 2.97 ± 0.09 |
| 3 × 10−6 | 81.3 ± 4.6 ** | 2.28 ± 0.18 * |
| 10−5 | 47.6 ± 4.6 *** ### | 2.43 ± 0.20 |
| 3 × 10−5 | 4.1 ± 1.9 *** ¥¥¥ | Nd |



3. Experimental
3.1. Chemicals
3.2. Animals
3.3. Preparation of Rat Aortic Rings
3.4. Experimental Protocols
3.4.1. Effect of FGAL on Rat Aorta Pre-Contracted with Phe or PMA
3.4.2. Effect of FGAL on Phenylephrine-Induced Cumulative Contractions
3.4.3. Effect of FGAL on Rat Aorta Pre-Contracted with KCl (30 or 80 mM)
3.4.4. Effect of FGAL on Rat Aorta Pre-Contracted with Phe in Both Absence and Presence of K+ Channel Blockers
3.4.5. Effect of FGAL on CaCl2-Induced Cumulative Contractions in Depolarizing Medium (80 mM KCl) Nominally Ca2+-free
3.4.6. Effect of FGAL on CaCl2-Induced Cumulative Contractions in the Presence of Verapamil and Phe
3.4.7. Effect of FGAL on Phe-Sensitive Ca2+ Mobilization from Sarcoplasmic Reticulum (SR)
3.4.8. Effect of Phosphodiesterase (PDE) Inhibitors on Rat Aorta Pre-Contracted with Phe in Both Absence and Presence of FGAL
3.5. Statistical Analysis
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 473–494. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Lira, D.P.; Dias, T.L.M.F.; Souza, E.T.; Matta, C.B.B.; Aquino, A.B.; Cavalcante‑Silva, L.H.A.; Silva, D.J.C.; Mella, E.A.C.; Agra, M.F.; et al. The antinociceptive and anti‑inflammatory activities of Piptadenia stipulacea Benth. (Fabaceae). J. Ethnopharmacol. 2010, 128, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Aggio, A.; Onori, L.; Croce, G.; Tiberti, S.; Ferri, C.; Ferri, L.; Desideri, G. Tea, flavonoids and nitric oxide-mediated vascular reactivity. J. Nutr. 2008, 138, 1554S–1560S. [Google Scholar] [PubMed]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Des. 2009, 15, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Mladenka, P.; Zatloukalová, I.; Filipsky, T.; Hrdina, R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, J.T.; Almeida, J.R.G.S.; Barbosa-Filho, J.M.; Assis, T.S.; Silva, M.S.; Cunha, E.V.L.; Braz-Filho, R.; Silva, B.A. Spasmolytic action of diplotropin, a furanoflavon from Diplotropis ferruginea Benth., involves calcium blockade in guinea-pig ileum. Z. Naturforsch. B 2005, 60, 1093–1100. [Google Scholar]
- Sato, Y.; He, J.X.; Nagai, H.; Tani, T.; Akao, T. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol. Pharm. Bull. 2007, 30, 145–149. [Google Scholar] [CrossRef]
- Elsohly, H.N.; El-Feraly, F.S.; Joshi, A.S.; Walker, L.A. Antiviral flavonoids from Alkanna orientalis. Planta Med. 1997, 63, 384. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, C.L.; Vasconcelos, L.H.C.; Correia, A.C.C.; Martins, I.R.R.; Lira, D.P.; Santos, B.V.O.; Silva, B.A. Spasmolytic effect of galetin 3,6-dimethyl ether, a flavonoid obtained from Piptadenia stipulacea (Benth) Ducke. J. Smooth Muscle Res. 2011, 47, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Török, J. Histamine-induced relaxation in pulmonary artery of normotensive and hypertensive rats: Relative contribution of prostanoids, nitric oxide and hyperpolarization. Physiol. Res. 2000, 49, 107–114. [Google Scholar] [PubMed]
- Edwards, G.; Félétou, M.; Weston, A.H. Endothelium-derived hyperpolarizing factors and associated pathways: A synopsis. Pflug. Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef]
- Chen, G.P.; Ye, Y.; Li, L.; Yang, Y.; Qian, A.B.; Hu, S.J. Endothelium-independent vasorelaxant effect of sodium ferulate on rat thoracic aorta. Life Sci. 2009, 84, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Jimenez, R.; Villar, I.C.; Perez-Viscaino, F.; Jimenez, J.; Tamargo, J. Vasorelaxant effects of the bioflavonoid chrysin in isolated rat aorta. Planta Med. 2001, 67, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Picq, M.; Dubois, M.; Prigent, A.F.; Nemoz, G.; Pacheco, H. Inhibition of the different cyclic nucleotide phosphodiesterase isoforms separated from rat brain by flavonoid compounds. Biochem. Int. 1989, 18, 47–57. [Google Scholar] [PubMed]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, unclouped eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Scholz, E.P.; Zitron, E.; Katus, H.A.; Karle, C.A. Cardiovascular ion channels as a molecular target of flavonoids. Cardiovasc. Ther. 2010, 28, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Frazziano, G.; Briones, A.M.; Cobeno, L.; Moreno, L.; Lodi, F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc. Res. 2007, 73, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.K.; White, C.R.; Pozzo-Miller, L.; Zhou, F.; Constance, C.; Inoue, T.; Patel, R.P.; Parks, D.A. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic. Biol. Med. 2010, 49, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003, 27, 201–206. [Google Scholar] [PubMed]
- Baraban, J.M.; Gould, R.J.; Peroutka, S.J.; Snyder, S.H. Phorbol ester effects on neurotransmission: Interaction with neurotransmitters and calcium in smooth muscle. Proc. Natl. Acad. Sci. USA 1985, 82, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.M.; Flaim, S.F. Phorbol ester contracts rabbit thoracic aorta by increasing intracellular calcium and by activating calcium influx. Biochem. Biophys. Res. Commun. 1986, 138, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Alioua, A.; Mahajan, A.; Nishimaru, K.; Zarei, M.M.; Stefani, E.; Toro, L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist‑induced vasoconstriction. Proc. Natl. Acad. Sci. USA 2002, 99, 14560–14565. [Google Scholar] [CrossRef] [PubMed]
- May, L.T.; Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric modulation of G protein‑coupled receptors. Ann. Rev. Pharmacol. Toxicol. 2007, 47, 1–51. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [PubMed]
- Gurney, A.M. Mechanisms of drug-induced vasodilation. J. Pharm. Pharmacol. 1994, 46, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.A.; Han, J.; Jung, I.D.; Park, W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008, 44, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Fang, L.H.; Li, Y.J.; Du, G.H. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vasc. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Álvarez, E.; Campos‑Toimil, M.; Justiniano‑Basaran, H.; Lugnier, C.; Orallo, F. Study of the mechanisms involved in the vasorelaxation induced by (‒)-epigallocatechin-3-gallate in rat aorta. Br. J. Pharmacol. 2006, 147, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Iozzi, D.; Schubert, R.; Kalenchuk, V.U.; Neri, A.; Sgaragli, G.; Fusi, F.; Saponara, S. Quercetin relaxes rat tail main artery partly via a PKG-mediated stimulation of KCa1.1 channels. Acta Physiol. 2013, 208, 329–339. [Google Scholar] [CrossRef]
- Nelson, M.T.; Huang, Y.; Brayden, J.E.; Hescheler, J.; Standen, N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 1990, 344, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Guibert, C.; Marthan, R.; Savineau, J.P. Angiotensin II-induced Ca2+-oscillations in vascular myocytes from the rat pulmonary artery. Am. J. Physiol. 1996, 270, L637–L642. [Google Scholar] [PubMed]
- Guibert, C.; Ducret, T.; Savineau, J.P. Voltage-independent calcium influx in smooth muscle. Prog. Biophys. Mol. Biol. 2008, 98, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Menice, C.B.; Laporte, R.; Morgan, K.G. Mechanisms of smooth muscle contraction. Physiol. Rev. 1996, 76, 967–1003. [Google Scholar] [PubMed]
- Noguera, M.A.; Ivorra, M.D.; Chuliá, S.; D’ocon, P. Capacitative Ca2+ entry associated with α1‑adrenoceptors in rat aorta. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 356, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Morello, S.; Vellecco, V.; Alfieri, A.; Mascolo, N.; Cicala, C. Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Sci. 2006, 78, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Sammels, E.; Parys, J.B.; Missiaen, L.; Smedt, H.D.; Bultynck, G. Intracelular Ca2+ storage in health and disease: A dynamic equilibrium. Cell Calcium 2010, 47, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Ghiasi, S.; Azimi, H.; Fakhari, S.; Abdollahi, M. A review of the herbal phosphodiesterase inhibitors: Future perspective of new drugs. Cytokine 2010, 49, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, H.; Kasuya, Y.; Kamata, K. Effects of calmodulin antagonist (W-7) on phorbol ester (PMA)-induced contractile response in isolated rat aorta. J. Smooth Muscle Res. 2001, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dunne, A. Comparison of individual and cumulative dose-response curves [proceedings]. Br. J. Pharmacol. 1979, 67, 491P–492P. [Google Scholar] [PubMed]
- Niu, L.G.; Zhang, M.S.; Liu, Y.; Xue, W.X.; Liu, D.B.; Zhang, J.; Liang, Y.Q. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur. J. Pharmacol. 2008, 580, 169–174. [Google Scholar] [PubMed]
- Mishra, S.K.; Aaronson, P.I. A role for a glibenclamide-sensitive, relatively ATP-insensitive K+ current, in regulating membrane potential and current in rat aorta. Cardiovasc. Res. 1999, 44, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C.; Clement-Chomienne, O.; Aiello, E.A. Regulation of 4‑aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem. Cell Biol. 1996, 74, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Van Der Staay, F.J.; Fanelli, R.J.; Blokland, A.; Schmidt, B.H. Behavioral effects of apamin, a selective inhibitor of the SKCa-channel, in mice and rats. Neurosci. Biobehav. Rev. 1999, 23, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.J.R.; Hodgson, W.C.; Sikorski, B.W.; King, R.G. Attenuated responses to endothelin‑1, KCl and CaCl2, but not noradrenaline, of aortae from rats with streptozotocin‑induced diabetes mellitus. Br. J. Pharmacol. 1991, 104, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Senejoux, F.; Girard, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Demougeot, C. Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J. Ethnopharmacol. 2010, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, D.H.; Barnard, E.A.; Hoyer, D.; Humphrey, P.P.A.; Leff, P.; Shankley, N.P. International Union of Pharmacology Committee on receptor nomenclature and drug classification. IX. Recommendations on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 1995, 47, 255–266. [Google Scholar] [PubMed]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on receptor nomenclature and drug classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of galetin 3,6-dimethyl ether are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macêdo, C.L.; Vasconcelos, L.H.C.; De C. Correia, A.C.; Martins, I.R.R.; De Lira, D.P.; De O. Santos, B.V.; De A. Cavalcante, F.; Da Silva, B.A. Mechanisms Underlying Vasorelaxation Induced in Rat Aorta by Galetin 3,6-Dimethyl Ether, a Flavonoid from Piptadenia stipulacea (Benth.) Ducke. Molecules 2014, 19, 19678-19695. https://doi.org/10.3390/molecules191219678
Macêdo CL, Vasconcelos LHC, De C. Correia AC, Martins IRR, De Lira DP, De O. Santos BV, De A. Cavalcante F, Da Silva BA. Mechanisms Underlying Vasorelaxation Induced in Rat Aorta by Galetin 3,6-Dimethyl Ether, a Flavonoid from Piptadenia stipulacea (Benth.) Ducke. Molecules. 2014; 19(12):19678-19695. https://doi.org/10.3390/molecules191219678
Chicago/Turabian StyleMacêdo, Cibério L., Luiz H. C. Vasconcelos, Ana C. De C. Correia, Italo R. R. Martins, Daysianne P. De Lira, Bárbara V. De O. Santos, Fabiana De A. Cavalcante, and Bagnólia A. Da Silva. 2014. "Mechanisms Underlying Vasorelaxation Induced in Rat Aorta by Galetin 3,6-Dimethyl Ether, a Flavonoid from Piptadenia stipulacea (Benth.) Ducke" Molecules 19, no. 12: 19678-19695. https://doi.org/10.3390/molecules191219678