The Remarkable Structural Diversity Achieved in ent-Kaurane Diterpenes by Fungal Biotransformations
Abstract
:1. Introduction
| Compound | Type | Plant or fungi (biotransformation) source | Biological activities | Reference |
|---|---|---|---|---|
| ent-18-acetoxykaur-16-ene | NP | Annona squamosa | anti-inflammatory and analgesic | [19] |
| ent-2α,16β,17-trihydroxykauran-19-oic acid and ent-3α,16β,17-trihydroxykauran-19-oic acid | NP | Mikania hirsutissima | immunomodulatory (human lymphocytes) | [20] |
| ent- 3β,15β,18-trihydroxykaur-16-ene | BP | Mucor plumbeus (fungus) | anti-allergic | [21] |
| ent 7β,16β,17-trihydroxykauran-6-one and ent-7α,16β,17-trihydroxykauran-6-one | NP | Broussonetia papyrifera | anti-tyrosinase | [22] |
| ent-13,16β,17-trihydroxykauran-19-oic acid | BP | Mucor recurvatus (fungus) | glucocorticoid agonists | [23] |
| ent-3β,15β,18-trihydroxykaur-16-ene ent-3-oxo-15β,18-dihydroxykaur-16-ene and ent-3β,15β-dihydroxykaur-16-ene | NP | Suregada multiflora | anti-allergic | [24] |
| ent-16βH,17-isobutyryloxykauran-19-oic acid and ent-16βH,17-acetoxy-18-isobutyryloxykauran-19-oic acid | NP | Siegesbeckia glabrescens | antidiabetic and antiobesity | [25] |
| ent-2α-hydroxy,16-oxo-17-norkauran-19-oic acid | BP | Fusarium proliferatum (fungus) | allelopathic | [26] |
| ent-7α,11β-dihydroxykaur-16-en-19-oic acid and ent-1β,7α-dihydroxykaur-16-en-19-oic acid | BP | Aspergillus niger (fungus) | spasmolytic | [27] |
| ent-16β,19-dihydroxykaurane and ent-16β,17,19-trihydroxykaurane | BP | Cephalosporium aphidicola (fungus) | allelopathic | [28] |
| ent-kaur-16-en-19-oic acid (kaurenoic acid) | NP | Aspilia foliacea | antimicrobial | [29] |
| NP | Mikania obtusata, Xylopia frutescens, X. sericea and Wedelia paludosa | trypanocidal | [30,31] | |
| NP | Melantheria albinervia | larvicidal | [32] | |
| NP | Annona glabra | antimicrobial, antifungal, antihelmintic and sporicidal | [33] | |
| NP | W. paludosa | antinociceptive | [34] | |
| NP | Copaifera langsdorffii | cytotoxic and genotoxic | [35,36] | |
| NP | W. paludosa | anti-inflammatory | [37] | |
| NP | Laetia thamnia | anti-Parkinsonism | [38] |



2. Biotransformations of Kaurane Diterpenes by Fungi from Gibberella fujikuroi Complex

3. Biotransformation Scope

4. Most Common Methylene Hydroxylation in Kaurane Diterpenes
| Fungal species | Positions and stereochemistry | References |
|---|---|---|
| Absidia blakesleeana | 7β, 11α, 13 | [64] |
| Aspergillus niger | 3α, 7β, 11α | [23,27,65] |
| Aspergillus ochraceus | 6β, 7α, 13 | [66,67] |
| Calonectria decora | 7α, 7β | [66,67] |
| Cephalosporium aphidicola | 3α, 11β | [68,69,70] |
| Cunninghamella bainieri | 9 | [43] |
| Cunninghamellha blakesleeana | 7β | [71] |
| Fusarium fujikuroi | 3α, 3β, 6β, 7β | [60] |
| Fusarium moniliforme | 11β | [72] |
| Gibberella fujikuroi | 3β, 6α, 6β, 7α, 7β, 9, 11β, 13 | [10,53,55,56,57,58,61,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] |
| Mucor plumbeus | 6α, 7α, 9, 11β | [21,101,102] |
| Mucor recurvatus | 7β, 11α, 11β | [23] |
| Psylocybe cubensis | 11β | [103] |
| Rhizopus nigricans | 3α, 7α, 7β, 13 | [59,62,66,67] |
| Rhizopus oligosporus | 7β, 9 | [64] |
| Rhizopus stolonifer | 7β, 9, 11β | [101,104,105] |
| Verticillium lecanii | 7α, 7β, 11β | [31] |







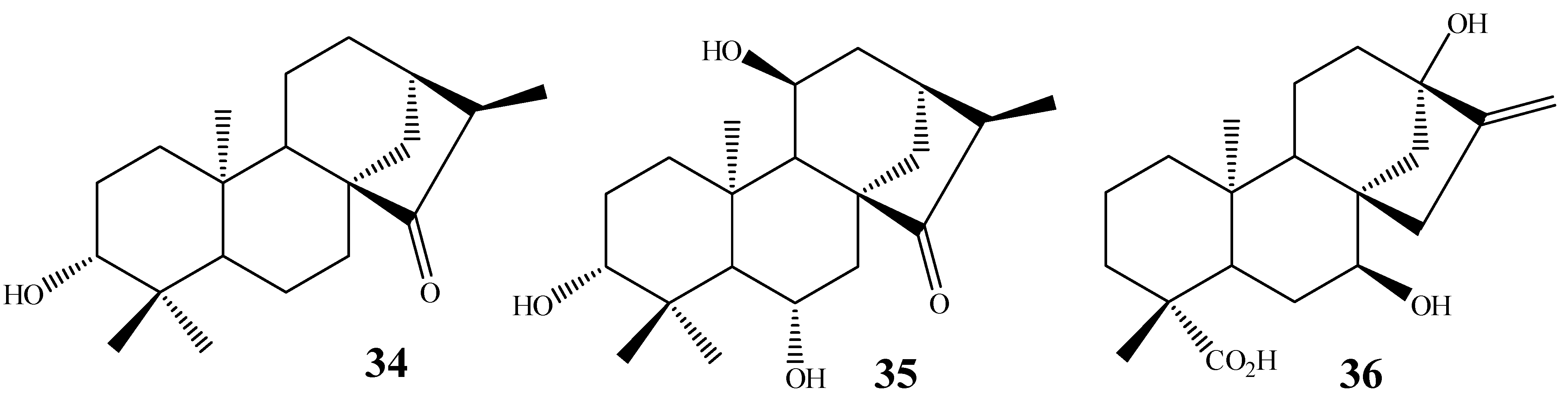
| Substrate | Fungus | Positions hydroxylated | Yield [66] (%) | Yield [67] (%) |
|---|---|---|---|---|
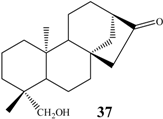 ent-19-hydroxy-16-oxo-17-nor-kaurane | A. ochraceus | 7α | n.i. | 20 |
| C.decora | 1α 7α | 10 10 | 10 10 | |
| R. nigricans | 1α 7α | 20 20 | 20 20 | |
 ent-16-oxo-17-nor-kauran-19-oic acid | A. ochraceus | 13 | 5 | 5 |
| C. decora | 1α 7α 7β | 10 15 40 | 5 15 40 | |
| R. nigricans | 1α 7α 7β | 30 30 5 | 30 30 5 | |
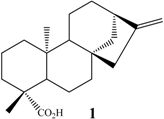 ent-kaur-16-en-19-oic acid | A. ochraceus | 16α, 17 | 20 | 10 |
| C. decora | 7α, 15α 15α 7α | 30 5 5 | 30 5 5 | |
| R. nigricans | 16α, 17 7β | n.i. 25 | 10 25 | |
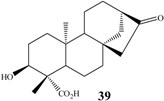 ent-3α-hydroxy-16-oxo-17-nor-kauran-19-oic acid | A. ochraceus | 6β 7α | 30 25 | n.i. n.i. |
| C. decora | 7α | 40 | n.i. | |
| R. nigricans | 1α 7α | 25 35 | n.i. n.i. |
5. Hydroxylations on Ring D





6. Some Unconventional Biotransformations




7. Hydroxylations of Carbons 9 and 13


8. Hydroxylation of Kaurane Diterpene Methyl Groups




9. Structural Alterations Other than Hydroxylation





10. Final Remarks
Acknowledgments
Conflicts of Interest
References
- Leresche, J.E.; Meyer, H.-P. Chemocatalysis and Biocatalysis (Biotransformation): Some thoughts of a chemist and of a biotechnologist. Org. Process Res. Dev. 2006, 10, 572–580. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavors and fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef]
- Schwab, W.; Fuchs, C.; Huang, F.C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013, 115, 3–8. [Google Scholar] [CrossRef]
- Prieto, S.; Gloria, A.; Perea, V.; Janeth, A.; Ortiz, L.; Claudia, C. Microbial biotransformation of (R)-(+)-limonene by Penicillium digitatum DSM 62840 for producing (R)-(+)-terpineol. Vitae 2011, 18, 136–172. [Google Scholar]
- Barrero, A.F.; Oltra, J.E.; Raslan, D.S.; Saúde, D.A. Microbial transformation of sesquiterpene lactones by the fungi Cunninghamella echinulata and Rhizopus oryzae. J. Nat. Prod. 1999, 62, 726–729. [Google Scholar] [CrossRef]
- Rocha, B.A.; Pupo, M.T.; Antonucci, G.A.; Sampaio, S.V.; Paiva, R.D.M.A.; Said, S.; da Costa, F.B. Microbial transformation of the sesquiterpene lactone tagitinin C by the fungus Aspergillus terreus. J. Ind. Microbiol. Biotechnol. 2012, 39, 1719–1724. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.R.; Takahashi, J.A. Rearrangement and oxidation of β-amyrin promoted by growing cells of Lecanicillium muscarinium. Nat. Prod. Res. 2010, 24, 767–774. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Aranda, C.; Kurina, M.; Rodríguez, J.A.; Theoduloz, C. Biotransformations of imbricatolic acid by Aspergillus niger and Rhizopus nigricans cultures. Molecules 2007, 12, 1092–1100. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez-Vallejo, V.; Guillermo, R. On the biotransformation of ent-trachylobane to ent-kaur-11-ene diterpenes. J. Nat. Prod. 2011, 74, 1985–1989. [Google Scholar] [CrossRef]
- Fraga, B.M.; Bressa, C.; González-Vallejo, V.; González, P.; Guillermo, R. Biotransformation of ent-kaur-16-ene and ent-trachylobane 7β-acetoxy derivatives by the fungus Gibberella fujikuroi (Fusarium fujikuroi). Phytochemistry 2012, 81, 60–70. [Google Scholar] [CrossRef]
- Hanson, J.R.; Reese, P.B.; Takahashi, J.A.; Wilson, M.R. Biotransformation of some stemodane diterpenoids by Cephalosporium aphidicola. Phytochemistry 1994, 36, 1391–1393. [Google Scholar] [CrossRef]
- Hanson, J.R.; Jarvis, A.G.; Laboret, F.; Takahashi, J. The incubation of 3α,16β-dihydroxyaphidicolane with Cephalosporium aphidicola. Phytochemistry 1995, 38, 73–75. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, P.; Hernández, M.G.; Chamy, M.C.; Garbarino, J.A. Microbiological transformation of an ent-pimaradiene hydrocarbon by Gibberella fujikuroi. Phytochem. Lett. 2009, 2, 201–203. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Artega, J.M.; Suárez, S. The microbiological transformation of the diterpenes dehydroabietanol and teideadiol by Mucor plumbeus. Phytochemistry 2003, 63, 663–668. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez, P.; Gonzalez-Vallejo, V.; Guillermo, R.; Diaz, L.N. Biotransformation of 7α-hydroxy- and 7-oxo-ent-atis-16-ene derivatives by the fungus Gibberella fujikuroi. Phytochemistry 2010, 71, 1313–1321. [Google Scholar] [CrossRef]
- Oliveira, B.H.; Strapasson, R.A. Biotransformation of isosteviol by Fusarium verticilloides. Phytochemistry 1996, 43, 393–395. [Google Scholar] [CrossRef]
- Hu, S.; Tian, X.; Zhu, W.; Fang, Q. Biotransformation of 2α,5α,10β,14β-Tetraacetoxy-4(20),11-taxadiene by the Fungi Cunninghamella elegans and Cunninghamella echinulata. J. Nat. Prod. 1996, 59, 1003–1009. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. The biological activity of natural occurring kaurane diterpenes. Fitoterapia 1997, 68, 303–325. [Google Scholar]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of 18-acetoxy-ent-kaur-16-ene from Annona squamosa L. bark. Inflammopharmacol. 2011, 19, 111–115. [Google Scholar] [CrossRef]
- Ohkoshi, E.; Kamo, S.; Makino, M.; Fujimoto, Y. ent-Kaurenoic acids from Mikania hirsutissima (Compositae). Phytochemistry 2004, 65, 885–890. [Google Scholar] [CrossRef]
- Fraga, B.M.; Alfonso, I.; Gonzalez-Vallejo, V.; Guillermo, R. Microbial transformation of two 15α-hydroxy-ent-kaur-16-ene diterpenes by Mucor plumbeus. Tetrahedron 2010, 66, 227–234. [Google Scholar] [CrossRef]
- Ko, H.-H.; Chang, W.-L.; Lu, T.-M. Antityrosinase and antioxidant effects of ent-kaurane diterpenes from leaves of Broussonetia papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef]
- Yang, L.-M.; Hsu, F.-L.; Chang, S.-F.; Cheng, J.-T.; Hsu, C.-Y.; Liu, P.-C.; Lin, S.-J. Microbial metabolism of steviol and steviol-16α,17-epoxide. Phytochemistry 2007, 68, 562–570. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Yodsaoue, O.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; TewtrakuL, S.; Kanjana-opas, A. Potential anti-allergic ent-kaurene diterpenes from the bark of Suregada multiflora. Phytochemistry 2006, 67, 2630–2634. [Google Scholar] [CrossRef]
- Kim, S.; Na, M.; Oh, H.; Jang, J.; Sohn, C.B.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. PTP1B inhibitory activity of kaurane diterpenes isolated from Siegesbeckia glabrescens. J. Enzyme Inhib. Med. Chem. 2006, 21, 379–383. [Google Scholar] [CrossRef]
- Rocha, A.D.; dos Santos, G.C.; Fernandes, N.G.; Pfenning, L.H.; Takahashi, J.A.; Boaventura, M.A.D. Hydroxylation at carbon-2 of ent-16-oxo-17-norkauran-19-oic acid by Fusarium proliferatum. J. Nat. Prod. 2010, 73, 1431–1433. [Google Scholar] [CrossRef]
- Marquina, S.; Parra, J.L.; González, M.; Zamilpa, A.; Escalante, J.; Trejo-Hernández, M.R.; Álvarez, L. Hydroxylation of the diterpenes ent-kaur-16-en-19-oic and ent-beyer-15-en-19-oic acids by the fungus Aspergillus niger. Phytochemistry 2009, 70, 2017–2022. [Google Scholar] [CrossRef]
- Rocha, D.; Takahashi, J.A.; Boaventura, M.A.D. Di- and tri-hydroxylated kaurane derivatives from microbial transformation of ent-kaur-16-en-19-ol by Cephalosporium aphidicola and their allelopathic activity on Lactuca sativa (Lettuce). Eclet. Quim. 2009, 34, 57–62. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Furtado, N.A.; de Oliveira, D.C.; da Costa, F.B.; Martins, C.H.; de Carvalho, T.C.; Porto, T.S.; Veneziani, R.C. Antimicrobial activity of kaurane diterpenes against oral pathogens. Z. Naturforsch. 2008, 63, 326–330. [Google Scholar]
- Alves, T.M.A.; Chaves, P.P.G.; Santos, L.M.S.T.; Nagem, T.J.; Murta, S.M.F.; Ceravolo, I.P.; Romanha, A.J.; Zani, C.L. A diterpene from Mikania obtusata active on Trypanosoma cruzi. Planta Med. 1995, 61, 85–87. [Google Scholar] [CrossRef]
- Vieira, H.S.; Takahashi, J.A.; Boaventura, M.A.D. Novel derivatives of ent-17,19-dihydroxy-16βH-kaurane obtained by biotransformation with Verticillium lecanii. J. Agric. Food Chem. 2002, 50, 3704–3707. [Google Scholar] [CrossRef]
- Slimestad, R.; Marston, A.; Mavi, S.; Hostettmann, K. Larvicidal constituents of Melantheria albinervia. Planta Med. 1995, 61, 562–563. [Google Scholar] [CrossRef]
- Padmaja, V.; Thankamany, V.; Hara, N.; Fujimoto, Y.; Hisham, A. Biological activities of Annona glabra. J. Ethnopharmacol. 1995, 48, 21–24. [Google Scholar] [CrossRef]
- Block, L.C.; Santos, A.R.S.; de Souza, M.M.; Scheidt, C.; Yunes, R.A.; Santos, M.A.; Monache, F.D.; Cechinel-Filho, V. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J. Ethnopharmacol. 1998, 61, 85–89. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Cunha, G.M.A.; Farias, P.A.M.; Viana, G.S.B.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Gramosa, N.V.; Rao, V.S.N. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon 2002, 40, 1231–1234. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Costa-lotufo, L.V.; Moraes, M.O.; Burbano, R.R.; Silveira, E.R.; Cunha, K.M.A.; Rao, V.S.N.; Moura, D.J.; Rosa, R.M.; Henriques, J.A.P.; et al. Genotoxicity evaluation of kaurenoic acid, a bioactive diterpenoid present in copaiba oil. Food Chem. Toxicol. 2006, 44, 388–392. [Google Scholar] [CrossRef]
- De Carli, R.B.G.; Siqueira, P.R.A.; Kaiser, M.L.; Freitas, R.A.; de Souza, M.M.; Cechinel-Filho, V.; Lucinda-Silva, R.M. Topical anti-inflammatory effect of creams containing kaurenoic acid isolated from Wedelia paludosa in Mice. Lat. Am. J. Pharm. 2009, 28, 594–598. [Google Scholar]
- Guillopé, R.; Escobar-Khondiker, M.; Guérineau, V.; Laprévote, O.; Höglinger, G.U.; Champy, P. Kaurenoic acid from pulp of Annona cherimolia in regard to Annonaceae-induced Parkinsonism. Phytother. Res. 2011, 25, 1861–1864. [Google Scholar] [CrossRef]
- Chang, F.-R.; Yang, P.-Y.; Lin, J.-Y.; Lee, K.-H.; Wu, Y.-C. Bioactive kaurane diterpenoids from Annona glabra. J. Nat. Prod. 1998, 61, 437–439. [Google Scholar] [CrossRef]
- Paiva, L.A.; Gurgel, L.A.; Silva, R.M.; Tome, A.R.; Gramosa, N.V.; Silveira, E.R.; Santos, F.A.; RAO, V.S. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffii on acetic acid-induced colitis in rats. Vascul. Pharmacol. 2002, 39, 303–307. [Google Scholar] [CrossRef]
- akahashi, J.A.; Vieira, H.S.; Silva, E.A.; Boaventura, M.A.D.; Oliveira, A.B.; Chiari, E. Preparation and activity of diterpenoids against trypomastigotes of Trypanosoma cruzi. Rev. Bras. Farmacogn. 2002, 12 (supl. 1), 118–120. [Google Scholar]
- Zhang, Y.-H.; Peng, H.-Y.; Xia, G.-H.; Wang, M.-Y.; Han, Y. Anticancer effect of two diterpenoid compounds isolated from Annona glabra Linn. Acta Pharmacol. Sin. 2004, 25, 937–942. [Google Scholar]
- Chang, S.-F.; Yang, L.-M.; Hsu, F.-L.; Hsu, J.-Y.; Liaw, J.-H.; Lin, S.-J. Transformation of steviol-16α,17-epoxide by Streptomyces griseus and Cunninghamella bainieri. J. Nat. Prod. 2006, 69, 1450–1455. [Google Scholar] [CrossRef]
- Sun, H.-D.; Huang, S.-X.; Han, Q.-B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vásquez, L. A Structure-activity study of antibacterial diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef]
- Fujita, E.; Nagao, Y.; Kaneko, K.; Nakazawa, S.; Kuroda, H. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem. Pharm. Bull. 1976, 24, 2118–2127. [Google Scholar] [CrossRef]
- Lin, L.; Gao, Q.; Cui, C.; Zhao, H.; Fu, L.; Chen, L.; Yang, B.; Luo, W.; Zhao, M. Isolation and identification of ent-kaurane-type diterpenoids from Rabdosia serra (MAXIM.) HARA leaf and their inhibitory activities against HepG-2, MCF-7, and HL-60 cell lines. Food Chem 2012, 131, 1009–1014. [Google Scholar] [CrossRef]
- Nagao, Y.; Ito, N.; Kohno, T.; Kuroda, H.; Fujita, E. Antitumor activity of Rabdosia and Teucrium diterpenoids against P 388 lymphocytic leukemia in mice. Chem. Pharm. Bull. 1982, 30, 727–729. [Google Scholar] [CrossRef]
- Dutta, T.K.; Samanta, T.B. Bioconversion of progesterone by the activated immobilized conidia of Aspergillus ochraceus. Curr. Microbiol. 1999, 39, 309–312. [Google Scholar] [CrossRef]
- Lisurek, M; Bernhardt, R. Modulation of aldosterone and cortisol synthesis on the molecular level. Mol. Cell. Endocrinol. 2004, 215, 149–159. [Google Scholar] [CrossRef]
- Kvas, M.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Steenkamp, E.T. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 2009, 34, 1–21. [Google Scholar]
- Ávalos, J.; Cerdá-Olmedo, E.; Reyes, F.; Barrero, A.F. Gibberellins and other metabolites of Fusarium fujikuroi and related fungi. Curr. Org. Chem. 2007, 11, 721–737. [Google Scholar] [CrossRef]
- Fraga, B.M.; Bressa, C.; González, P.; Guillermo, R.; Hernández, M.G.; Suárez, S. The microbiological transformation of 7α-hydroxy-ent-kaur-16-ene derivatives by Gibberella fujikuroi. Phytochemistry 2007, 68, 1557–1563. [Google Scholar]
- Hedden, P. The oxidases of gibberellin biosynthesis: Their function and mechanism. Physiol. Plant. 1997, 101, 709–719. [Google Scholar]
- Alam, M.; Hanson, J.R.; Sarah, F. The biotransformation of some 6-substituted ent-kaur-16-enes by Gibberella fujikuroi. Phytochemistry 1991, 30, 807–809. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez, P.; Guillermo, R.; Hanson, J.R.; Hernandez, M.G.; Takahashi, J.A. The microbiological transformation of two ent-16β,17-epoxykaurane derivatives by Gibberella fujikuroi. Phytochemistry 1994, 37, 717–721. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez, P.; Guillermo, R.; Hernandez, M.G.; Perales, A. The chemical and microbiological preparation of 15-oxo-gibberellin derivatives. Tetrahedron 1995, 51, 10053–10064. [Google Scholar] [CrossRef]
- Fraga, B.M.; Gonzalez, P.; Hernandez, M.G.J.; Suárez, S. Biotransformation of 7-oxo-ent-kaur-16-ene derivatives by Gibberella fujikuroi. Tetrahedron 2005, 61, 5623–5632. [Google Scholar] [CrossRef]
- García-Granado, A.; Martinez, A.; Onorato, M.E.; Ruiz, M.L.; Sánches, J.M.; Arias, J.M. Biotransformation of highly substituted ent-kaur-16-enes by Rhizopus nigricans. Phytochemistry 1990, 29, 121–126. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Vallejo, V.; Guillermo, R.; Amaro-Luis, J.M. Microbiological transformation of two 15α-hydroxy-ent-kaur-9(11),16-diene derivatives by the fungus Fusarium fujikuroi. Phytochemistry 2013, 89, 39–46. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Guillermo, R. The biotransformation of two 3,15-oxygenate ent-kaurane derivatives by Gibberella fujikuroi. J. Nat. Prod. 1996, 59, 952–957. [Google Scholar] [CrossRef]
- García-Granado, A.; Martinez, A.; Ortiz, A.; Onorato, M.E.; Arias, J.M. Microbiological transformation of tetracyclic diterpenes: Conversion of ent-kaurenones by Curvularia and Rhizopus strains. J. Nat. Prod. 1990, 53, 441–450. [Google Scholar] [CrossRef]
- Giles, P.M. Revised section F: Natural products and related compounds. Pure Appl. Chem. 1999, 71, 587–643. [Google Scholar] [CrossRef]
- Taveepanich, S.; Muangsin, N.; Saithong, S.; Pakawatchai, C.; Chaichit, N.; Roengsumran, S.; Petsom, A. Biotransformation of ent-kaur-16-en-19-oic acid by Absidia blakesleeana and Rhizopus oligosporus. Nat. Prod. Rep. 2010, 24, 1050–1058. [Google Scholar] [CrossRef]
- Anderson, A.B.; McCrindle, R.; Turnbull, J.K. Microbiological transformation of 17-norkauran-16-one and 16-norphyllocladan-16-one by Aspergillus niger. J. Chem. Soc. Chem. Comm. 1973, 143–144. [Google Scholar]
- Beilby, J.P.; Ghisalberti, E.L.; Jefferies, P.R.; Sefton, M.A.; Sheppard, P.N. Microbiological transformation of tetracyclic diterpenes. Tetrahedron Lett. 1973, 14, 2589–2592. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Jefferies, P.R.; Sefton, M.A.; Sheppard, P.N. Microbial transformation of 19-oxigenated ent-kauranes. Tetrahedron 1977, 33, 2451–2456. [Google Scholar] [CrossRef]
- Boaventura, M.A.D.; Hanson, J.R.; Hitchcock, P.B.; Takahashi, J.A. The biotransformation of ent-19-hydroxykaur-16-en-15-one by Cephalosporium aphidicola. Phytochemistry 1994, 37, 387–389. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Hanson, J.R.; Takahashi, J.A. The biotransformation of ent-15-oxo-kaur-16-en-19-oic acid and its methyl ester by Cephalosporium aphidicola. Phytochemistry 1995, 40, 439–442. [Google Scholar] [CrossRef]
- Hanson, J.R.; Hitchcock, P.B.; Takahashi, J.A. Biotransformation of ent-16β,19-dihydroxykaurane by Cephalosporium aphidicola. Phytochemistry 1995, 40, 797–800. [Google Scholar] [CrossRef]
- El-Emary, N.A.; Kusano, G.; Takemoto, T. Microbial transformation of (−)-kaur-16-en-19-oic acid. Chem. Pharm. Bull. 1976, 24, 1664–1667. [Google Scholar] [CrossRef]
- Oliveira, B.H.; Filho, J.D.F.; Leal, P.C. Biotransformation os steviol by Aspergillus niger and Fuscarium moniliforme. J. Braz. Chem. Soc. 2005, 16, 210–213. [Google Scholar]
- Hanson, J.R.; White, A.F. The transformation of steviol by Gibberella fujikuroi. Tetrahedron 1968, 24, 6291–9293. [Google Scholar] [CrossRef]
- Hanson, J.R.; Hawker, J.; White, A.F. Studies in terpenoid biosynthesis. Part IX. The sequence of oxidation on ring B in kaurene-gibberellin biosynthesis. J. Chem. Soc. Perkin Trans. 1 1972, 1892–1895. [Google Scholar] [CrossRef]
- Cook, I.F.; Jefferies, P.R.; Knox, J.R. Acidic ent-kauranoids from the metabolism of ent-kaura-2,16-dien-19-ol in Gibberella fujikuroi. Tetrahedron 1975, 31, 251–255. [Google Scholar] [CrossRef]
- Bearder, J.R.; MacMillan, J.; Wels, C.M. The metabolism of steviol to 13-hydroxylated ent-gibberellanes and ent-kauranes. Phytochemisty 1975, 14, 1741–1748. [Google Scholar] [CrossRef]
- Wada, K.; Imai, T.; Shibata, K. Microbial productions of unnatural gibberellins from (−)-kaurene derivatives in Gibberella fujikuroi. Agric. Biol. Chem. 1970, 43, 1157–1158. [Google Scholar] [CrossRef]
- Wada, K.; Yamashita, H. Synthesis and microbial transformation of ent-12β-hydroxykaur-16-ene. Agric. Biol. Chem. 1980, 44, 2249–2250. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hanson, J.R.; Hernández, M.G.; Sarah, F.Y. The microbiological transformation of some ent-kaur-16-ene 7-,15- and 18-alcohols by Gibberella fujikuroi. Phytochemistry 1980, 19, 1087–1091. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, A.G.; Hanson, J.R.; Hernández, M.G. The microbiological transformation of some ent-3β-hydroxykaur-16-enes by Gibberella fujikuroi. Phytochemistry 1981, 20, 57–61. [Google Scholar] [CrossRef]
- Wada, K.; Imai, T.; Yamashita, H. Microbiological production of plant gibberellins and related compounds from ent-kaurane derivatives in Gibberella fujikuroi. Agric. Biol. Chem. 1981, 45, 1833–1842. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, A.G.; González, P.; Hanson, J.R.; Hernández, M.G.; Hitchcock, P.B. The formation of ent-6α,7α-epoxykaurene of possible biosynthetic significance by Gibberella fujikuroi. J. Chem. Soc. Chem. Comm. 1982, 311–312. [Google Scholar]
- Beale, M.H.; Bearder, J.R.; Down, G.H.; Hutchison, M.; MacMillan, J.; Phinney, B.O. The biosynthesis of kaurenolide diterpenoids by Gibberella fujikuroi. Phytochemistry 1982, 21, 1279–1287. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Murofushi, N.; Takahashi, N. Structures of the metabolites from steviol methyl ester by Gibberella fujikuroi. Agric. Biol. Chem. 1982, 46, 2313–2318. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, A.G.; González, P.; Hanson, J.R.; Hernández, M.G. The microbiological transformation of some ent-kaur-6,16-dienes by Gibberella fujikuroi. Phytochemistry 1983, 22, 691–694. [Google Scholar] [CrossRef]
- Gaskin, P.; Hutchison, M.; Lewis, N.; MacMillan, J.; Phinney, B.O. Microbiological conversion of 12-oxygenated and other derivatives of ent-kaur-16-en-19-oic acid by Gibberella fujikuori. Phytochemistry 1984, 23, 559–564. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, P.; Hernández, M.G.; García-Tellado, F.; Perales, A. The microbiological transformation of candidiol, ent-15β,18-dihydroxy-kaur-16-ene, by Gibberella fujikuroi. Phytochemistry 1986, 25, 1235–1237. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Rodrigues, M.D.; Diaz, C.E.; González, P.; Hanson, J.R. Transformation of ent-kaur-15-ene by Gibberella fujikuroi. Phytochemistry 1987, 26, 1931–1934. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Diaz, C.E.; González, P.; Guillermo, R. The microbiological transformation of some ent-15β-hydroxykaurenes by Gibberella fujikuroi. Phytochemistry 1988, 27, 3131–3136. [Google Scholar] [CrossRef]
- Hanson, J.R.; Oliveira, B.H. The microbiological transformation of steviol derivatives by Rhyzopus nigricans and Gibberella fujikuroi. Phytochemistry 1990, 29, 3805–3807. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; González, P. The microbiological transformation of some ent-7α,15β-dihydroxykaurene derivatives by Gibberella fujikuroi. Phytochemistry 1991, 30, 2567–2571. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; González, P. Biotransformation of two ent-15β-hydroxy-kaur-16-ene derivatives by Gibberella fujikuroi. Phytochemistry 1992, 31, 3845–3849. [Google Scholar] [CrossRef]
- Fraga, B.M.; García-Tellado, F.; González, P.; Hernández, M.G. The chemical and microbiological synthesis of 14-hydroxy-gibberellins. Tetrahedron 1992, 48, 8491–8504. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; García-Tellado, F.; González, P.; Perales, A. The biotransformation of two ent-15β-16β-epoxy-kaurane derivatives by Gibberella fujikuroi. Phytochemistry 1993, 34, 133–138. [Google Scholar] [CrossRef]
- Fraga, B.M.; García-Tellado, F.; González, P.; Hernández, M.G. The microbiological transformation of 14β,19-dihydroxy-ent-kaur-15-ene by Gibberella fujikuroi. Phytochemistry 1993, 34, 1035–1040. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, P.; Guillermo, R.; Hernández, M.G. A study of microbiological reduction of α,β-unsaturated carbonyl ent-kaurenes by Gibberella fujikuroi. Tetrahedron 1996, 52, 13767–13782. [Google Scholar] [CrossRef]
- Fraga, B.M.; González, P.; Guillermo, R.; Hernández, M.G. The formation of an ent-11α,16α-epoxykaurene of biosynthetic significance by Gibberella fujikuroi. Nat. Prod. Lett. 1996, 8, 257–262. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Cabrera, E.; Reyes, F.; Álvarez, M. Metabolism of gibberellins and ent-kaurenoids in mutants of Gibberella fujikuroi. Phytochemistry 1999, 50, 1133–1140. [Google Scholar]
- Barrero, A.F.; Oltra, J.E.; Cerdá-Olmedo, E.; Ávalos, J.; Justicia, J. Microbial transformation of ent-kaurenoic acid and its 15-hydroxy derivatives by the SG138 mutant of Gibberella fujikuroi. J. Nat. Prod. 2001, 64, 222–225. [Google Scholar]
- Fraga, B.M.; Guillermo, R.; Hernández, M.G. The microbiological transformation of two 15β-hydroxy-ent-kaurene diterpenes by Gibberella fujikuroi. J. Nat. Prod. 2004, 67, 64–69. [Google Scholar] [CrossRef]
- Boaventura, M.A. D.; Oliveira, A.B.; Hanson, J.R.; Hitchcock, P.B.; Takahashi, J.A. The biotransformation of methyl ent-15-oxokaur-16-en-19-oate by Rhizopus stolonifer and Mucor plumbeus. Phytochemistry 1995, 40, 1667–1669. [Google Scholar] [CrossRef]
- Fraga, B.M.; Álvarez, L.; Suárez, S. Biotransformation of the diterpenes epicandicandiol and candicandiol by Mucor plumbeus. J. Nat. Prod. 2003, 66, 327–331. [Google Scholar] [CrossRef]
- Pechwang, J.; Sihanonth, P.; Pornpakakul, S.; Muangsin, N.; Piapukiew, J.; Vangnai, A.; Chaichit, N.; Chuchawankul, S.; Petsom, A. Biotransformation of ent-kaur-16-en-19-oic acid by Psilocybe cubensis. Nat. Prod. Rep. 2010, 24, 905–914. [Google Scholar] [CrossRef]
- Silva, E.A.; Takahashi, J.A.; Boaventura, M.A.D.; Oliveira, A.B. The biotransformation of ent-kaur-16-en-19-oic acid by Rhizopus stolonifer. Phytochemistry 1999, 52, 397–400. [Google Scholar] [CrossRef]
- Vieira, H.S.; Takahashi, J.A.; Boaventura, M.A.D. Biotransformation of methyl ent-17-hydroxy-16β-kauran-19-oate by Rhizopus stolonifer. Appl. Microbiol. Biotechnol. 2000, 53, 601–604. [Google Scholar] [CrossRef]
- Hueso-Falcón, I.; Cuadrado, I.; Cidre, F.; Amaro-Luis, J.M.; Ravelo, Á.G.; Estevez-Braun, A.; Heras, B.; Hortelano, S. Synthesis and anti-inflammatory activity of ent-kaurene derivates. Eur. J. Med. Chem. 2011, 46, 1291–1305. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Frade, R.F.M.; Afonso, C.A.M. Isolation, chemical, and biotransformation routes of labdane-type diterpenes. Chem. Rev. 2011, 111, 4418–4452. [Google Scholar] [CrossRef]
- Silva, E.O.; Furtado, N.A.J.C.; Aleu, J.; Collado, I.G. Terpenoid biotransformations by Mucor species. Phytochem. Rev. 2013, 12, 857–876. [Google Scholar] [CrossRef]
- Dewick, P.M. The Mevalonate and Methylerythritol Phosphate Pathaways: Terpenoids and Steroids. In Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 187–310. [Google Scholar]
- Sticht, G.; Käferstein, H. Detection of psilocin in body fluids. Forensic. Sci. Int. 2000, 113, 403–407. [Google Scholar] [CrossRef]
- Kamata, T.; Katagi, M.; Tsuchihashi, H. Metabolism and toxicological analyses of hallucinogenic tryptamine analogues being abused in Japan. Forensic Toxicol. 2010, 28, 1–8. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoea, D.; Williams, T.; Stonea, J.M.; Reeda, L.J.; Colasantia, A.; Tyackea, R.J.; Leechd, R.; Maliziab, A.L.; Murphye, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Tanaka, N.; Ooba, N.; Duan, H.; Takaishi, Y.; Nakanishi, Y.; Bastow, K.; Lee, K. Kaurene and abietane diterpenoids from Tripterygium doianum (Celastraceae). Phytochemistry 2004, 65, 2071–2076. [Google Scholar] [CrossRef]
- Topçu, G.; Ertaş, A.; Öztürk, M.; Dinçel, D.; Kılıç, T.; Halfon, B. Ent-kaurane diterpenoids isolated from Sideritis congesta. Phytochem. Lett. 2011, 4, 436–439. [Google Scholar] [CrossRef]
- Hanson, J.R. The microbiological transformation of diterpenoids. Nat. Prod. Rep. 1992, 9, 139–151. [Google Scholar] [CrossRef]
- Milagre, H.M.S.; Martins, L.R.; Takahashi, J.A. Novel agents for enzymatic and fungal hydrolysis of stevioside. Braz. J. Microbiol. 2009, 40, 367–372. [Google Scholar] [CrossRef]
- Rojas, M.C.; Urrutia, O.; Cruz, C.; Gaskin, P.; Tudzynski, B.; Hedden, P. Kaurenolides and fujenoic acids are side products of the gibberellins P450-1 monooxygenase in Gibberella fujikuroi. Phytochemistry 2004, 65, 821–830. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, J.A.; Gomes, D.C.; Lyra, F.H.; Dos Santos, G.F.; Martins, L.R. The Remarkable Structural Diversity Achieved in ent-Kaurane Diterpenes by Fungal Biotransformations. Molecules 2014, 19, 1856-1886. https://doi.org/10.3390/molecules19021856
Takahashi JA, Gomes DC, Lyra FH, Dos Santos GF, Martins LR. The Remarkable Structural Diversity Achieved in ent-Kaurane Diterpenes by Fungal Biotransformations. Molecules. 2014; 19(2):1856-1886. https://doi.org/10.3390/molecules19021856
Chicago/Turabian StyleTakahashi, Jacqueline A., Dhionne C. Gomes, Fernanda H. Lyra, Gabriel F. Dos Santos, and Leonardo R. Martins. 2014. "The Remarkable Structural Diversity Achieved in ent-Kaurane Diterpenes by Fungal Biotransformations" Molecules 19, no. 2: 1856-1886. https://doi.org/10.3390/molecules19021856






