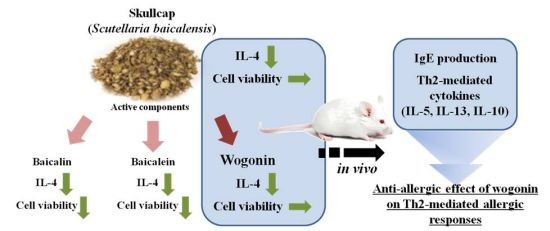
Skullcap (Scutellaria baicalensis) Extract and Its Active Compound, Wogonin, Inhibit Ovalbumin-Induced Th2-Mediated Response
Abstract
:1. Introduction

2. Results
2.1. Effects of Skullcap Extract on the OVA-Induced Th2 Immune Response in Ex Vivo

2.2. Effects of Baicalein, Baicalin, and Wogonin on the OVA-Induced Th2 Dominant Response

2.3. Effects of Wogonin by Oral Gavage on OVA-Induced Immune Responses In Vivo

3. Discussion
4. Experimental
4.1. Materials
4.2. Animals
4.3. Sensitization and Challenge with OVA and Preparation of Splenocyte Cultures
4.4. Schedules for Mice Sensitization, OVA Challenges, and Sample Treatment In Vivo

4.5. Measurement of Cytokine Levels by ELISA
4.6. Cytotoxicity/Viability Assay
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, B.; Kang, S.; Jeong, G.; Lee, J.S.; Yu, Y.B.; Chun, M. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J. Ethnopharmacol. 2009, 126, 320–331. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Gwak, N.G.; Im, Y.S.; Lee, K.Y.; Sohn, Y.; Choi, H.; Yang, W.M. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J. Ethnopharmacol. 2012, 141, 345–349. [Google Scholar] [CrossRef]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; Shon, D.H. Inhibitory effect of skullcap (Scutellaria. baicalensis) extract on ovalbumin permeation in vitro and in vivo. Food Chem. 2013, 140, 22–30. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Y.; Chen, F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Malbalirajan, U.; Ahmad, T.; Rehman, R.; Leishangthem, G.D.; Dinda, A.K.; Agrawal, A.; Ghosh, B.; Sharma, S.K. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. Separation methods used for Scutellaria baicalensis active components. PLoS One 2013, 8, e62916. [Google Scholar]
- Guo, M.; Zhang, N.; Li, D.; Liang, D.; Liu, Z.; Li, F.; Fu, Y.; Cao, Y.; Deng, X.; Yang, Z. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int. Immunopharmacol. 2013, 16, 125–130. [Google Scholar] [CrossRef]
- Yeh, C.H.; Shih, H.C.; Hong, H.M.; Lee, S.S.; Yang, M.L.; Chen, C.J.; Kuan, Y.H. Protective effect of wogonin on proinflammatory cytokine generation via Jak1/3-STAT1/3 pathway in lipopolysaccharide stimulated BV2 microglial cells. Toxicol. Ind. Health 2013, 2013. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and naicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Hall, K.; Ha, T.; Li, C.; Krishnaswamy, G.; Chi, D.S. Baicalein inhibits IL-1β-and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin. Mol. Allergy 2007, 5, 5. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Wu, J.; Liu, B.; Gong, W.; Lv, Y.; Luo, Q.; Dun, X.; Dong, J. Effects of balicalin on airway remodeling in asthmatic mice. Planta Med. 2013, 79, 199–206. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Inhibition of contact dermatitis in animal models and suppression of proinflammatory gene expression by topically applied flavonoid, wogonin. Arch. Pharm. Res. 2004, 27, 442–448. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Chu, Y.; Li, M. Identification of Baicalin as an immunoregulatory compound by controlling T(H)17 cell differentiation. PLoS One 2011, 6, e17164. [Google Scholar] [CrossRef]
- Chow, J.M.; Shen, S.C.; Wu, C.Y.; Chen, Y.C. 12-o-Tetradecanoylphorbol 13-acetate prevents baicalein-induced apoptosis via activation of protein kinase C and JNKs in human leukemia cells. Apoptosis 2006, 11, 1999–2011. [Google Scholar] [CrossRef]
- Debes, G.F.; Diehl, M.C. CCL8 and skin T cells—An allergic attraction. Nat. Immunol. 2011, 12, 111–112. [Google Scholar] [CrossRef]
- Endo, Y.; Iwamura, C.; Kuwahara, M.; Suzuki, A.; Sugaya, K.; Tumes, D.J.; Tokoyoda, K.; Hosokawa, H.; Yamashita, M.; Nakayama, M. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity 2011, 35, 733–745. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The bioloy of IgE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Burrow, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef]
- Crestani, E.; Lohman, I.C.; Guerra, S.; Wright, A.L.; Halonen, M. Association of IL-5 cytokine production and in vivo IgE levels in infants and parents. J. Allergy Clin. Immun. 2007, 120, 820–826. [Google Scholar] [CrossRef]
- Losol, P.; Kim, S.H.; Hwang, E.K.; Shin, Y.S.; Park, H.S. IL-5 Promoter polymorphism enhances IgE responses to staphylococcal superantigens in adult asthmatics. Allergy Asthma Immunol. Res. 2013, 5, 106–109. [Google Scholar] [CrossRef]
- Sample Availability: All samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shin, H.S.; Bae, M.-J.; Choi, D.W.; Shon, D.-H. Skullcap (Scutellaria baicalensis) Extract and Its Active Compound, Wogonin, Inhibit Ovalbumin-Induced Th2-Mediated Response. Molecules 2014, 19, 2536-2545. https://doi.org/10.3390/molecules19022536
Shin HS, Bae M-J, Choi DW, Shon D-H. Skullcap (Scutellaria baicalensis) Extract and Its Active Compound, Wogonin, Inhibit Ovalbumin-Induced Th2-Mediated Response. Molecules. 2014; 19(2):2536-2545. https://doi.org/10.3390/molecules19022536
Chicago/Turabian StyleShin, Hee Soon, Min-Jung Bae, Dae Woon Choi, and Dong-Hwa Shon. 2014. "Skullcap (Scutellaria baicalensis) Extract and Its Active Compound, Wogonin, Inhibit Ovalbumin-Induced Th2-Mediated Response" Molecules 19, no. 2: 2536-2545. https://doi.org/10.3390/molecules19022536