Limonoids from the Fruits of Khaya ivorensis
Abstract
:1. Introduction

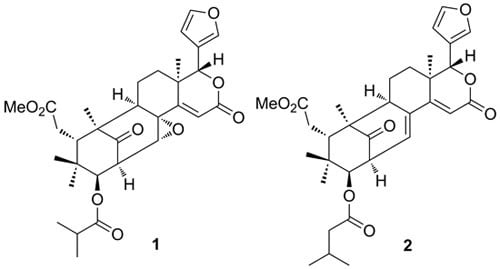
2. Results and Discussion
| 1 | 2 | |||
|---|---|---|---|---|
| δC, type a | δH (J in Hz) b | δC, type a | δH (J in Hz) b | |
| 1 | 214.8, s | 214.8, s | ||
| 2 | 49.7, d | 3.92 dd (9.3, 2.0) | 49.9, d | 3.94 ddd (9.0, 6.1, 1.3) |
| 3 | 77.6, d | 5.39 d (9.3) | 79.1, d | 5.14 d (9.2) |
| 4 | 40.3, s | 39.3, s | ||
| 5 | 42.6, d | 3.67 d (2.0) | 41.2, d | 3.57 dd (7.2, 4.7) |
| 6 | 33.6, t | 2.44 m | 33.5, t | 2.55 m |
| 7 | 174.3, s | 174.5, s | ||
| 8 | 61.5, s | 136.9, s | ||
| 9 | 56.3, d | 2.11 br s | 54.1, d | 2.46 m |
| 10 | 49.0, s | 52.5, s | ||
| 11 | 21.59, t | α 1.22 m, β 1.65 m | 22.1, t | α 1.81 m, β 1.66 m |
| 12 | 33.2, t | α 1.26 m, β 2.11 m | 32.9, t | α 1.19 m, β 1.95 m |
| 13 | 39.7, s | 38.0, s | ||
| 14 | 162.0, s | 161.1, s | ||
| 15 | 119.5, d | 6.66 s | 113.3, d | 6.57 s |
| 16 | 164.6, s | 165.3, s | ||
| 17 | 80.0, d | 5.57 s | 80.1, d | 5.20 s |
| 18 | 21.7, q | 1.22 s | 22.2, q | 1.08 s |
| 19 | 16.1, q | 1.13 s | 16.1, q | 1.30 s |
| 20 | 121.1, s | 121.6, s | ||
| 21 | 143.1, d | 7.88 br s | 142.6, d | 7.81 s |
| 22 | 111.4, d | 6.70 br s | 111.3, d | 6.66 d (1.0) |
| 23 | 144.2, d | 7.70 br s | 144.1, d | 7.67 t (1.6) |
| 28 | 22.9, q | 0.90 s | 23.0, q | 0.93 s |
| 29 | 21.60, q | 0.79 s | 21.4, q | 0.84 s |
| 30 | 62.7, d | 4.51 d (2.0) | 129.8, d | 6.55, dd (6.0, 2.9) |
| 7-OMe | 52.3, q | 3.65 s | 52.3, q | 3.69 s |
| 3-acyl-1' | 176.0, s | 172.7, s | ||
| 2' | 34.9, d | 2.81 m | 43.4, t | 2.35 m |
| 3' | 19.6, q | 1.26 d (7.1) | 26.0, d | 2.19 dt (13.7, 6.8) |
| 4' | 19.2, q | 1.23 d (7.1) | 22.82, q | 0.95 d (6.7) |
| 5' | 22.85, q | 0.92 d (6.7) | ||


| Compound | HL-60 | SMMC-7721 | A-549 | MCF-7 | SW480 |
|---|---|---|---|---|---|
| 1 | >40 | >40 | >40 | >40 | >40 |
| 2 | >40 | >40 | 37.3 | >40 | >40 |
| 3 | 21.2 | 21.1 | 23.8 | >40 | 32.6 |
| 4 | >40 | >40 | 39.5 | 26.1 | >40 |
| 5 | >40 | >40 | >40 | >40 | >40 |
| 6 | >40 | >40 | >40 | >40 | >40 |
| 7 | >40 | >40 | >40 | >40 | >40 |
| 8 | >40 | >40 | >40 | >40 | >40 |
| Cisplatin a | 1.1 | 4.5 | 6.6 | 13.1 | 11.1 |
3. Experimental
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Fang, X.; Di, Y.T.; Hao, X.J. The advances in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011, 15, 1363–1391. [Google Scholar] [CrossRef]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Chen, S.K.; Li, H.; Chen, B.Y. In Zhongguo Zhiwu Zhi; Science Press: Beijing, China, 1997; pp. 46–47. [Google Scholar]
- Zhang, B.; Wang, Y.; Yang, S.P.; Zhou, Y.; Wu, W.B.; Tang, W.; Zuo, J.P.; Li, Y.; Yue, J.M. Ivorenolide A, an unprecedented immunosuppressive macrolide from Khaya ivorensis: Structural elucidation and bioinspired total synthesis. J. Am. Chem. Soc. 2012, 134, 20605–20608. [Google Scholar]
- Agbedahunsi, J.; Fakoya, F.; Adesanya, S. Studies on the anti-inflammatory and toxic effects of the stem bark of Khaya ivorensis (Meliaceae) on rats. Phytomedicine 2004, 11, 504–508. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, S.P.; Yin, S.; Zhang, C.R.; Wu, Y.; Yue, J.M. Limonoids from Khaya ivorensis. Phytochemistry 2009, 70, 1305–1308. [Google Scholar] [CrossRef]
- Adesogan, E.K.; Taylor, D.A.H. Limonoid extractives from Khaya ivorensis. J. Chem. Soc. C 1970, 12, 1710–1714. [Google Scholar] [CrossRef]
- Adesida, G.; Adesogan, E.; Okorie, D.; Taylor, D.; Styles, B. The limonoid chemistry of the genus Khaya (Meliaceae). Phytochemistry 1971, 10, 1845–1853. [Google Scholar] [CrossRef]
- Taylor, D.A.H. The structure of an extractive from Khaya ivorensis. Phytochemistry 1977, 16, 1847–1849. [Google Scholar] [CrossRef]
- Vanucci, C.; Lange, C.; Lhommet, G.; Dupont, B.; Davoust, D.; Vauchot, B.; Clement, J.L.; Brunck, F. An insect antifeedant limonoid from seed of khaya ivorensis. Phytochemistry 1992, 31, 3003–3004. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Hashinaga, F.; Nakatani, M. Antifungal activity of limonoids from Khaya ivorensis. Pest Manag. Sci. 2005, 61, 186–190. [Google Scholar]
- Li, M.Y.; Yang, X.B.; Pan, J.Y.; Feng, G.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Granatumins A–G, limonoids from the seeds of a Krishna Mangrove, Xylocarpus granatum. J. Nat. Prod. 2009, 72, 2110–2114. [Google Scholar] [CrossRef]
- Gan, L.S.; Wang, X.N.; Wu, Y.; Yue, J.M. Tetranortriterpenoids from Cipadessa baccifera. J. Nat. Prod. 2007, 70, 1344–1347. [Google Scholar] [CrossRef]
- Siva, B.; Poornima, B.; Venkanna, A.; Prasad, K.R.; Sridhar, B.; Nayak, V.L.; Ramakrishna, S.; Babu, K.S. Methyl angolensate and mexicanolide-type limonoids from the seeds of Cipadessa baccifera. Phytochemistry 2014, 98, 174–182. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Iwagawa, T.; Doe, M.; Nakatani, M. Antifungal limonoids from the fruits of Khaya senegalensis. Fitoterapia 2004, 75, 566–572. [Google Scholar] [CrossRef]
- Akisanya, A.; Arene, E.; Bevan, C.; Ekong, D.; Nwaji, M.; Okogun, J.; Powell, J.; Taylor, D. West African timbers. Part XII. The inter-relation of gedunin and khivorin. J. Chem. Soc. C 1996, 506–509. [Google Scholar]
- Bickii, J.; Njifutie, N.; Foyere, J.A.; Basco, L.K.; Ringwald, P. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae). J. Ethnopharmacol. 2000, 69, 27–33. [Google Scholar] [CrossRef]
- Tchimene, M.K.; Ngamga, D.; Tane, P.; Sterner, O.; Connolly, J.D. 3,7-Dideacetyl-6 alpha-hydroxykhivorin, a new limonoid from Khaya senegalensis (Meliaceae). Bull. Chem. Soc. Ethiop. 2006, 20, 69–73. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–53. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, K.-L.; Liao, S.-G.; Zheng, X.-L.; Na, Z.; Hu, H.-B.; Zhang, P.; Xu, Y.-K. Limonoids from the Fruits of Khaya ivorensis. Molecules 2014, 19, 3004-3011. https://doi.org/10.3390/molecules19033004
Ji K-L, Liao S-G, Zheng X-L, Na Z, Hu H-B, Zhang P, Xu Y-K. Limonoids from the Fruits of Khaya ivorensis. Molecules. 2014; 19(3):3004-3011. https://doi.org/10.3390/molecules19033004
Chicago/Turabian StyleJi, Kai-Long, Shang-Gao Liao, Xiao-Ling Zheng, Zhi Na, Hua-Bin Hu, Ping Zhang, and You-Kai Xu. 2014. "Limonoids from the Fruits of Khaya ivorensis" Molecules 19, no. 3: 3004-3011. https://doi.org/10.3390/molecules19033004