Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]pyrimidin-4-one Derivatives
Abstract
:1. Introduction

2. Results and Discussion
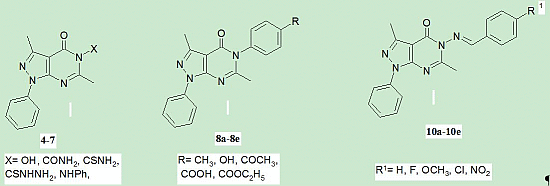
2.1. Chemical Synthesis


2.2. In Vitro Anticancer Screening
| Compound No. | IC50 in µM |
|---|---|
| Doxorubicin | 5 |
| 4 | 49 |
| 5a | 52 |
| 5b | 38 |
| 6 | 52 |
| 7 | 14 |
| 8a | 33 |
| 8b | 25 |
| 8c | 26 |
| 8d | 25 |
| 8e | 27 |
| 9 | 84 |
| 10a | 17 |
| 10b | 12 |
| 10c | 18 |
| 10d | 12 |
| 10e | 11 |
3. Experimental
3.1. General
3.1.1. General Procedure for the Synthesis of 3,6-Dimethyl-1-phenyl-5-substituted-1,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-ones 5a,b
3.1.2. General Procedure for the Synthesis of 3,6-Dimethyl-1-phenyl-5-(4-substitutedphenyl)-1,5-dihydropyrazolo[3,4-d]pyrimidin-4-ones 8a–e
3.1.3. General Procedure for the Synthesis of (E)-3,6-Dimethyl-5-(4-substitutedbenzylideneamino)-1-phenyl-1,5-dihydropyrazolo[3,4-d]pyrimidin-4-ones 10a–e
3.2. Biological Testing
3.2.1. Materials and Methods
3.2.2. Measutement of Potential Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Wu, H.; Chang, D.; Huang, C. Targeted therapy for cancer. J. Cancer Mol. 2006, 2, 57–66. [Google Scholar]
- Sierra, J.R.; Cepero, V.; Giordano, S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol. Cancer 2010, 9, 75–88. [Google Scholar] [CrossRef]
- Riley, L.B.; Desai, D.C. The molecular basis of cancer and the development of targeted therapy. Surg. Clin. N. Am. 2009, 89, 1–15. [Google Scholar] [CrossRef]
- Cheng, C.C.; Robins, R.K. Potential Purine Antagonists. VI. Synthesis of 1-alkyl- and 1-aryl-4-substituted pyrazolo[3,4-d]pyrimidines. J. Org. Chem. 1956, 21, 1240–1256. [Google Scholar] [CrossRef]
- Ismail, Z.H.; Abdel-Gawad, S.M.; Abdel-Aziem, A.; Ghorab, M.M. Synthesis of some new biologically active sulfur compounds containing pyrazolo[3,4-d]pyrimidine moiety. Phosphor. Sulfur Silicon 2003, 178, 1795–1805. [Google Scholar]
- Carraro, F.; Naldini, A.; Pucci, A.; Locatelli, G.A.; Maga, G.; Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Brullo, C.; et al. Pyrazolo[3,4-d]pyrimidines as potent antiproliferative and proapoptotic agents toward A431 and 8701-BC cells in culture via inhibition of c-Src phosphorylation. J. Med. Chem. 2006, 49, 1549–1561. [Google Scholar] [CrossRef]
- El-Enany, M.M.; Kamel, M.M.; Khalil, O.M.; El-Nassan, H.B. Synthesis and antitumor activity of novel 6-aryl and 6-alkylpyrazolo[3,4-d]pyrimidin-4-one derivatives. Eur. J. Med. Chem. 2010, 45, 5286–5291. [Google Scholar] [CrossRef]
- Abd El Hamid, M.K.; Mihovilovic, M.D.; El-Nassan, H.B. Synthesis of novel pyrazolo[3,4-d]pyrimidine derivatives as potential anti-breast cancer agents. Eur. J. Med. Chem. 2012, 57, 323–328. [Google Scholar] [CrossRef]
- Kandeel, M.M.; Mohamed, L.W.; Abd El Hamid, M.K.; Negmeldin, A.T. Design, synthesis, and antitumor evaluation of novel pyrazolo[3,4-d]pyrimidine derivatives. Sci. Pharm. 2012, 80, 531–545. [Google Scholar] [CrossRef]
- Peat, A.J.; Garrido, D.; Boucheron, J.A.; Schweiker, S.L.; Dickerson, S.H.; Wilson, J.R.; Wang, T.Y.; Thomson, S.A. Novel GSK-3 inhibitors with improved cellular activity. Bioorg. Med. Chem. Lett. 2004, 14, 2127–2130. [Google Scholar] [CrossRef]
- Kim, D.C.; Lee, Y.R.; Yang, B.S.; Shin, K.J.; Kim, D.J.; Chung, B.Y.; Yoo, K.H. Synthesis and biological evaluations of pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 inhibitors. Eur. J. Med. Chem. 2003, 38, 525–532. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Mosti, L.; Maga, G.; Crespan, E.; Carraro, F.; Manetti, F.; Tintori, C.; et al. Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur. J. Med. Chem. 2008, 43, 2665–2676. [Google Scholar] [CrossRef]
- Schenone, S.; Bruno, O.; Bondavalli, F.; Ranise, A.; Mosti, L.; Menozzi, G.; Fossa, P.; Manetti, F.; Morbidelli, L.; Trincavelli, L.; et al. 1-(2-Chloro-2-phenylethyl)6-methylthio-1H-pyrazolo[3,4-d]pyrimidines-4-amino substituted and their biological evaluation. Eur. J. Med. Chem. 2004, 39, 153–160. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef]
- Hassan, G.S.; Kadry, H.H.; Abou-Seri, S.M.; Ali, M.M.; Mahmoud, A.E. Synthesis and in vitro cytotoxic activity of novel pyrazolo[3,4-d]pyrimidines and related pyrazole hydrazones toward breast adenocarcinoma MCF-7 cell line. Bioorg. Med. Chem. 2011, 19, 6808–6817. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.; Wolf, J.; Heigener, D.; Wu, Y.L. Reviewing the safety of erlotinib in non small cell lung cancer. Expert Opin. Drug Saf. 2011, 10, 147–157. [Google Scholar] [CrossRef]
- Omar, H.A.; Sargeant, A.M.; Weng, J.-R.; Wang, D.; Kulp, S.K.; Patel, T.; Chen, C.-S. Pulmonary endothelial impairment during gefitinib therapy: A preliminary assessment with iodine-123-metaiodobenzylguanidine (1231-MIBG) scintigraphy. Open Lung Cancer J. 2011, 4, 957–968. [Google Scholar]
- Fang, L.; Barekati, Z.; Zhang, B.; Liu, Z.; Zhong, X. Targeted therapy in breast cancer: What’s new? Eur. J. Med. Sci. 2011, 141, 1–9. [Google Scholar]
- Bakr, R.B.; Abdelall, E.K.A.; Abdel-Hamid, M.K.; Kandeel, M.M. Design and synthesis of new EGFR-tyrosine kinase inhibitors containing pyrazolo[3,4-d]pyrimidine cores as anticancer agents. Bull. Pharm. Sci. Assiut Univ. 2012, 35, 1–16. [Google Scholar] [CrossRef]
- Heravi, M.M.; Nami, N.; Seifi, N.; Oskooie, H.A.; Hekmatshoar, R. Microwave-assisted synthesis of substituted pyrazoles and pyrazolo[3,4-d]thiopyrimidines. Phosphor. Sulfur Silicon 2006, 181, 591–599. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdellatif, K.R.A.; Abdelall, E.K.A.; Abdelgawad, M.A.; Ahmed, R.R.; Bakr, R.B. Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]pyrimidin-4-one Derivatives. Molecules 2014, 19, 3297-3309. https://doi.org/10.3390/molecules19033297
Abdellatif KRA, Abdelall EKA, Abdelgawad MA, Ahmed RR, Bakr RB. Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]pyrimidin-4-one Derivatives. Molecules. 2014; 19(3):3297-3309. https://doi.org/10.3390/molecules19033297
Chicago/Turabian StyleAbdellatif, Khaled R. A., Eman K. A. Abdelall, Mohamed A. Abdelgawad, Rasha R. Ahmed, and Rania B. Bakr. 2014. "Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]pyrimidin-4-one Derivatives" Molecules 19, no. 3: 3297-3309. https://doi.org/10.3390/molecules19033297