Structures of Two New Flavonoids and Effects of Licorice Phenolics on Vancomycin-Resistant Enterococcus Species
Abstract
:1. Introduction
2. Results and Discussion


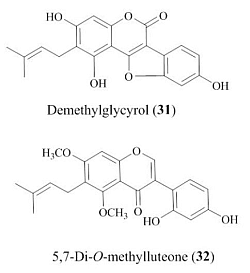
2.1. Structures of New Compounds

| Demethylglycyrol (31) | 5,7-Di-O-methylluteone (32) | |||||
|---|---|---|---|---|---|---|
| position | δC, type | δH (J in Hz) | HMBC a | δC, type | δH (J in Hz) | HMBC a |
| C-1 | 160.4, C | |||||
| C-2 | 113.9, C | 170.1, CH | 7.95, s | 3, 4, 1' | ||
| C-3 | 158.5, C | 119.9, C | ||||
| C-4 | 99.4, CH | 6.25, s | 2, 3, 4a, 11b | 178.8, C | ||
| C-4a | 156.4, C | 106.5, C | ||||
| C-5 | 159.2, C | |||||
| C-6 | 160.1, C | 115.6, C | ||||
| C-6a | 104.0, C | |||||
| C-6b | 114.9, C | |||||
| C-7 | 98.4, CH | 6.80, d (2.4) | 160.4, C | |||
| C-8 | 111.9, CH | 6.71, dd (2.4, 8.4) | 96.4, CH | 6.34, s | 6, 7, 4a, 8a | |
| C-8a | 156.7, C | |||||
| C-9 | 156.6, C | |||||
| C-10 | 120.0, CH | 7.25, d (8.4) | ||||
| C-10a | 156.1, C | |||||
| C-11a | 158.6, C | |||||
| C-11b | 104.1, C | |||||
| C-1' | 23.2, CH2 | 3.12, d (6.6) | 1, 2, 3, 2', 3' | 117.5, C | ||
| C-2' | 125.1, CH | 5.07, t (6.6) | 160.0, C | |||
| C-3' | 130.5, C | 128.2, CH | 8.02, d (8.4) | 2', 4' | ||
| C-4' | 25.8, CH3 | 1.60, s | 2',3', 5' | 157.1, C | ||
| C-5' | 17.9, CH3 | 1.77, s | 2', 3', 4' | 116.8, CH | 6.92, dd (2.4, 8.4) | 1' |
| C-6' | 103.3, CH | 6.82, d (2.4) | 2' | |||
| C-1'' | 23.5, CH2 | 3.38, d (6.6) | 5, 6, 7, 2'', 3'' | |||
| C-2'' | 125.2, CH | 5.14, t (6.6) | ||||
| C-3'' | 130.9, C | |||||
| C-4'' | 17.7, CH3 | 1.73, s | 2'', 3'' | |||
| C-5'' | 25.7, CH3 | 1.64, s | 2'', 3'' | |||
| 5-OCH3 | 61.3, CH3 | 3.78, s | 5 | |||
| 7-OCH3 | 55.9, CH3 | 3.43, s | 7 | |||

2.2. Antibacterial Effects of Licorice Phenolics on VRE
| Compounds | Bacterial Strains | |
|---|---|---|
| Enterococcus faecium FN-1 | Enterococcus faecalis NCTC12201 | |
| Flavonols and related compounds | ||
| Kaempferol-3- O-methyl ether (c) | >128 | >128 |
| Kaempferol ( 6) | >128 | >128 |
| Isolicoflavonol ( 7) | >128 | 128 |
| Flavanones | ||
| 6"-O-Acetylliquiritin ( 11) | >128 | >128 |
| Liquiritin ( 12) | >128 | 128 |
| Liquiritigenin ( 19) | >128 | >128 |
| Chalcone | ||
| Isoliquiritin ( 23) | >128 | >128 |
| Isoflavones and Isoflavans | ||
| Allolicoisoflavone B ( 2) | >128 | 128 |
| Formononetin ( 10) | >128 | >128 |
| Semilicoisoflavone B ( 14) | 32 | 64 |
| 5,7-Di- O-Methylluteone (32) | 64 | 128 |
| Gancaonin G ( 20) | 64 | 128 |
| 6,8-Diprenylorobol ( 22) | 128 | 128 |
| Glicoricone ( 25) | >128 | >128 |
| Licoricone ( 28) | 128 | >128 |
| Glyasperin D ( 29) | 32 | 64 |
| 3-Arylcoumarins | ||
| Isoglycycoumarin ( 8) | 64 | >128 |
| Licoarylcoumarin ( 9) | 16 | 16 |
| Glycyrin ( 16) | 16 | 32 |
| Glycycoumarin ( 26) | 16 | 16 |
| Coumestans | ||
| Glycyrol ( 15) | >128 | >128 |
| Isoglycyrol ( 18) | 32 | 64 |
| Demethlglycyrol ( 31) | 64 | 64 |
| 2-Arylcoumarones | ||
| Gancaonin I ( 17) | 8 | 16 |
| Licocoumarone ( 27) | 32 | 32 |
| Others | ||
| p-Hydroxybenzoic acid (13) | >128 | 128 |
| Standard antibacterial drugs | ||
| Erythromycin | >1024 | >1024 |
| Norfloxacin | >128 | 4 |
| Vancomycin | >100 | >100 |
| Linezolid | 2.5 | 2.5 |
| Imipenem | >64 | 2 |
| Tetracycline | 64 | 128 |
| Oxacillin | >1024 | 256 |
| Gentamicin | >1024 | >1024 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Methylation of Compound A and Glycyrol
3.6. Antibacterial Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharm. 2000, 46, 167–192. [Google Scholar] [CrossRef]
- Shen, X.-P.; Xiao, P.-G.; Liu, C.-X. Research and application of Radix Glycyrrhizae. Asian J. Pharmacodyn. Pharmacokin. 2007, 7, 181–200. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Messier, C.; Epifano, F.; Genovese, S.; Grenier, D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012, 18, 32–39. [Google Scholar] [CrossRef]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014. [Google Scholar] [CrossRef]
- Gafner, S.; Bergeron, C.; Villinski, J.R.; Godejohann, M.; Kessler, P.; Cardellina, J.H.; Grenier, D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: Antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011, 74, 2514–2519. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.Y. Antibacterial Compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti- Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef]
- Otsuka, N.; Liu, M.-H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef]
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoiso-avanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 48, 1286–1292. [Google Scholar] [CrossRef]
- McNeil, S.A.; Clark, N.M.; Chandrasekar, P.H.; Kauffman, C.A. Successful treatment of vancomycin-resistant Enterococcus faecium bacteremia with linezolid after failure of treatment with synercid (quinupristin/dalfopristin). Clin. Infect. Dis. 2000, 30, 403–404. [Google Scholar]
- Irani, M.; Sarmadi, M.; Bernard, F. Leaves Antimicrobial activity of Glycyrrhiza glabra L. Iran J. Pharm. Res. 2010, 9, 425–428. [Google Scholar]
- Badr, A.E.; Omar, N.; Badria, F.A. A laboratory evaluation of the antibacterial and cytotoxic effect of liquorice when used as root canal medicament. Int. Endod. J. 2011, 44, 51–58. [Google Scholar] [CrossRef]
- Fukai, T.; Oku, Y.; Hano, Y.; Terada, S. Antimicrobial activities of hydrophobic 2-Arylbenzofurans and an isoflavone against vancomycin-resistant Enterococci and methicillin-resistant Staphylococcus aureus. Planta Med. 2004, 70, 685–687. [Google Scholar] [CrossRef]
- Park, S.Y.; Lim, S.S.; Kim, J.K.; Kang, I.J.; Kim, J.S.; Lee, C.; Park, J.H.Y. Hexane-ethanol extract of Glycyrrhiza uralensis containing licoricidin inhibits the metastatic capacity of DU145 human prostate cancer cells. Br. J. Nutr. 2010, 104, 1272–1282. [Google Scholar] [CrossRef]
- Tahara, S.; Shibaki, S.; Ingham, J.L.; Mizutani, J. Further isoflavonoids from white lupin roots. Z. Naturforsch. C 1990, 45, 147–153. [Google Scholar]
- O’Neill, M.J.; Adesanya, S.A.; Roberts, M.F.; Inez, R.P. Inducible isoflavonoids from the lima bean, Phaseolus lunatus. Phytochemistry 1986, 25, 1315–1322. [Google Scholar] [CrossRef]
- Tahara, S.; Ingham, J.L.; Mizutani, J. Metabolites of 7-O-methylluteone from Botrytis cinerea. Nippon Nogei Kagaku Kaishi 1989, 63, 999–1007. [Google Scholar] [CrossRef]
- Valesi, A.G.; Rodriguez, E.; Vander Velde, G.; Mabry, T.J. Methylated flavonols in Larrea cuneifolia. Phytochemistry 1972, 11, 2821–2826. [Google Scholar]
- Xiao, Z.P.; Wu, H.K.; Wu, T.; Shi, H.; Hang, B.; Aisa, H.A. Kaempferol and quercetin flavonoids from Rosa rugosa. Chem. Nat. Compd. 2006, 42, 736–737. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; Chao, J.; Wu, J.; Wang, M. Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera). Food Chem. 2008, 106, 529–535. [Google Scholar] [CrossRef]
- Hatano, T.; Yasuhara, T.; Miyamoto, T.; Okuda, T. Anti-human immunodeficiency virus phenolics from licorice. Chem. Pharm. Bull. 1988, 36, 2286–2288. [Google Scholar] [CrossRef]
- Hatano, T.; Yasuhara, T.; Fukuda, T.; Noro, T.; Okuda, T. Phenolic constituents of licorice. II. Structures of licopyranocoumarin, licoarylcoumarin and glisoflavone, and glisoflavone, and inhibitory effects of licorice phenolics on xanthine oxidase. Chem. Pharm. Bull. 1989, 37, 3005–3009. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nair, M.G.; Santell, R.C. Microwave-mediated synthesis of anticarcinogenic isoflavones from soybeans. J. Agric. Food Chem. 1994, 42, 1869–1871. [Google Scholar] [CrossRef]
- Shen, F.J.; Hu, J.F.; Yu, Y.C.; Xu, Z.D. Studies on chemical constituents of Glycyrrhiza uralensis Fisch. Gaodeng Xuexiao Huaxue Xuebao 1995, 16, 574–574. [Google Scholar]
- Nakanishi, T.; Inada, A.; Kambayashi, K.; Yoneda, K. Flavonoid glycosides of the roots of Glycyrrhiza uralensis. Phytochemistry 1985, 24, 339–341. [Google Scholar] [CrossRef]
- Kiuchi, F.; Chen, X.; Tsuda, Y. Four new phenolic constituents from licorice (root of Glycyrrhiza sp.). Heterocycles 1990, 31, 629–636. [Google Scholar]
- Shul’ts, E.E.; Petrova, T.N.; Shakirov, M.M.; Chernyak, E.I.; Tolstikov, G.A. Flavonoids of roots of Glycyrrhiza uralensis growing in Siberia. Chem. Nat. Compd. 2000, 36, 362–368. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Wang, Q.H. Four new prenylated flavonoids from aerial parts of Glycyrrhiza uralensis. Heterocycles 1989, 29, 1761–1772. [Google Scholar] [CrossRef]
- Shiozawa, T.; Urata, S.; Kinoshita, T.; Saitoh, T. Revised structures of glycyrol and isoglycyrol, constituents of the root of Glycyrrhiza uralensis. Chem. Pharm. Bull. 1989, 37, 2239–2240. [Google Scholar] [CrossRef]
- Fukai, T.; Wang, Q.H.; Kitagawa, T.; Litaka, Y. Structures of six isoprenoids-substituted flavonoids, gancaonins F, G, R, I, glycyrol, and isoglycyrol from xibei licorice (Glycyrrhiza sp). Heterocycles 1989, 29, 1761–1772. [Google Scholar] [CrossRef]
- Krishnaswamy, N.R.; Seshadri, T.R.; Sharma, B.R. Study of partial demethylation of some polymethoxy-3-phenylcoumarins and preparation of some new members. Indian J. Chem. 1966, 4, 120–126. [Google Scholar]
- Nkengfack, A.E.; Sanson, D.R.; Fomum, Z.T.; Tempesta, M.S. 8-Prenylluteone, a prenylated isoflavone from Erythrina eriotriocha. Phytochemistry 1989, 28, 2522–2526. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- Singhal, A.K.; Sharma, R.P.; Thyagarajan, G.; Herz, W.; Govindan, S.V. New prenylated isoflavones and a prenylated dihydroflavonol from Millettia pachycarpa. Phytochemistry 1980, 9, 929–934. [Google Scholar]
- Hatano, T.; Fukuda, T.; Miyase, T.; Noro, T.; Okuda, T. Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase. Chem. Pharm. Bull. 1991, 39, 1238–1243. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Song, G.Q.; Jian, F.X.; Chang, X.R.; Guo, W.B. Chemical constituents of Glycyrrhiza uralensis Fisch—Structures of isolicoflavonol and glycycoumarin. Huaxue Xuebao 1984, 42, 1080–1084. [Google Scholar]
- Kaneta, M.; Saitoh, T.; Iitaka, Y.; Shibata, S. Chemical studies on the oriental plant drugs. XXXVI. Structure of licoricone, a new isoflavone from licorice root. Chem. Pharm. Bull. 1984, 21, 1338–1341. [Google Scholar]
- Kwon, H.J.; Kim, H.H.; Ryu, Y.B.; Kim, J.H.; Jeong, H.J.; Lee, S.W.; Lee, W.S. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioor. Med. Chem. 2010, 18, 7668–7674. [Google Scholar] [CrossRef]
- Sil Lee, Y.; Ha Kim, S.; Kyu Kim, J.; Shin, H.K.; Kang, Y.H.; Park, Y.; Lim, S.S. Rapid identification and preparative isolation of antioxidant components in licorice. J. Sep. Sci. 2010, 33, 664–671. [Google Scholar] [CrossRef]
- Sasaki, H.; Kashiwada, Y.; Shibatav, H.; Takaishi, Y. Prenylated flavonoids from the roots of Desmodium caudatum and evaluation of their antifungal activity. Planta Med. 2012, 78, 1851–1856. [Google Scholar] [CrossRef]
- Nagoshi, C.; Shiota, S.; Kuroda, T.; Shiota, S.; Hatano, T.; Yoshida, T.; Kariyama, R.; Tsuchiya, T. Synergistic effect of [10]-gingerol and aminoglycosides against vancomycin-resistant Enterococci (VRE). Biol. Pharm. Bull. 2006, 29, 443–447. [Google Scholar] [CrossRef]
- Hossion, A.M.; Zamami, Y.; Kandahary, R.K.; Tsuchiya, T.; Ogawa, W.; Iwado, A.; Sasaki, K. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. J. Med. Chem. 2011, 54, 3686–3703. [Google Scholar] [CrossRef]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 2002, 73, 536–539. [Google Scholar]
- Ohno, H.; Araho, D.; Uesawa, Y.; Kagaya, H.; Ishihara, M.; Sakagami, H.; Yamamoto, M. Evaluation of cytotoxiciy and tumor-specificity of licorice flavonoids based on chemical structure. Anticancer Res. 2013, 33, 3061–3068. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 6, 9, 12, 13, 16, 19 and 27 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eerdunbayaer; Orabi, M.A.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of Two New Flavonoids and Effects of Licorice Phenolics on Vancomycin-Resistant Enterococcus Species. Molecules 2014, 19, 3883-3897. https://doi.org/10.3390/molecules19043883
Eerdunbayaer, Orabi MAA, Aoyama H, Kuroda T, Hatano T. Structures of Two New Flavonoids and Effects of Licorice Phenolics on Vancomycin-Resistant Enterococcus Species. Molecules. 2014; 19(4):3883-3897. https://doi.org/10.3390/molecules19043883
Chicago/Turabian StyleEerdunbayaer, Mohamed A. A. Orabi, Hiroe Aoyama, Teruo Kuroda, and Tsutomu Hatano. 2014. "Structures of Two New Flavonoids and Effects of Licorice Phenolics on Vancomycin-Resistant Enterococcus Species" Molecules 19, no. 4: 3883-3897. https://doi.org/10.3390/molecules19043883