The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin
Abstract
:1. Introduction

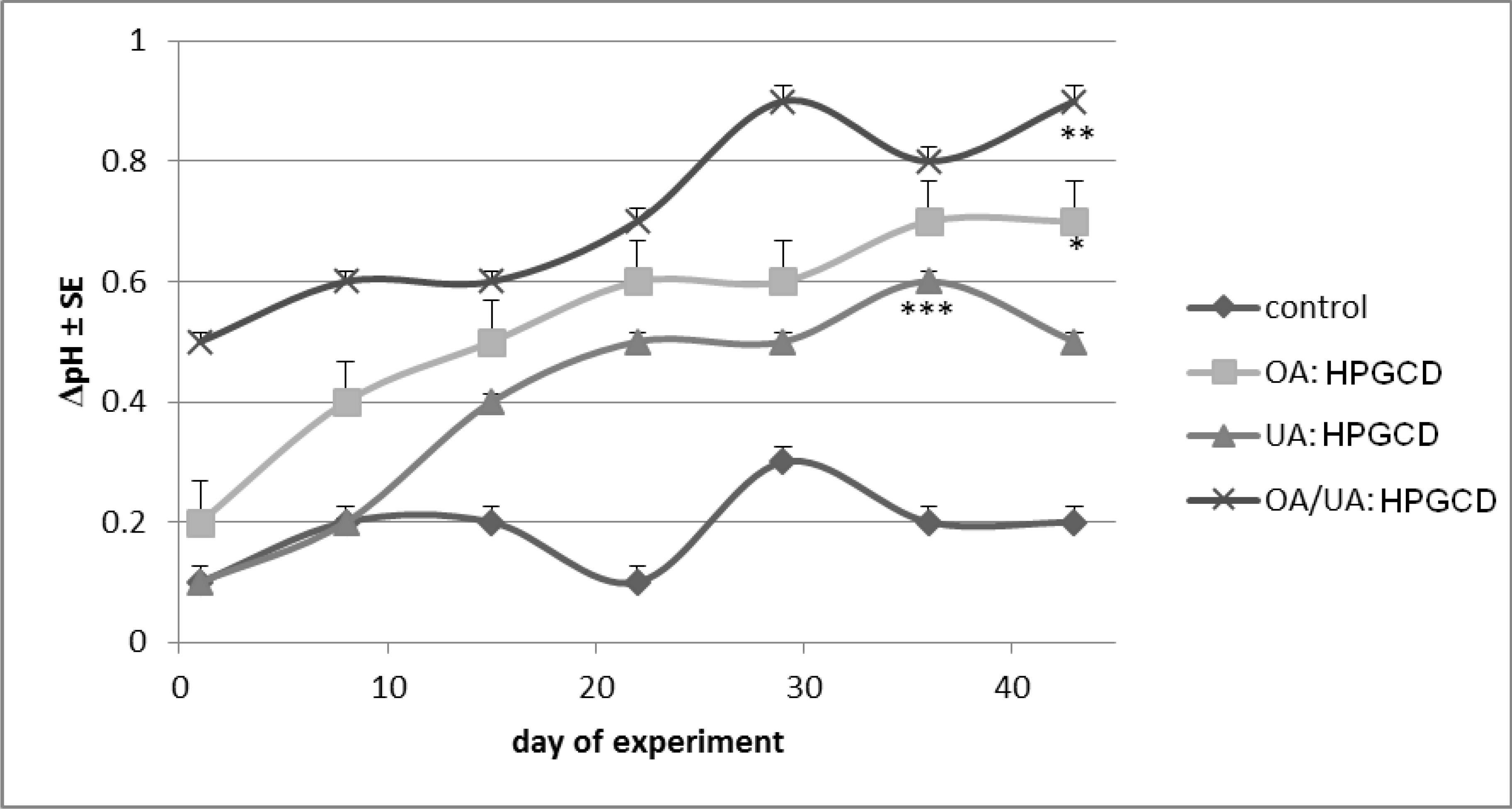
2. Results and Discussion











3. Experimental
3.1. Alamar Blue in Vitro Analysis
3.2. Preparation of Inclusion Complexes
3.3. In Vivo Experimental Cancer Procedure
3.4. Non-Invasive Skin Measurements
3.5. Statistical Analysis
3.6. Compliance with Ethics Requirements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dewick, P.M. The Mevalonate. and Deoxyxylulose. Phosphate Pathways: Terpenoids. and Steroids. In Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Esperandim, V.R.; Marçal, M.G.; Neres, N.B.D.R.; Cunha, N.L.; Andrade e Silva, M.L.; Cunha, W.R. Natural products and Chagas’ disease: The action of triterpenes acids isolated from Miconia species. Universitas. Scientiarum. 2013, 18, 243–256. [Google Scholar]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef]
- Huang, D.; Ding, Y.; Lia, Y.; Zhang, W.M.; Fang, W.S.; Chen, X.G. Anti-tumor activity of a 3-oxo derivative of oleanolic acid. Cancer Lett. 2006, 233, 289–296. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Takada, K.; Nakane, T.; Masuda, K.; Ishii, H. Ursolic acid and oleanolic acid, members of pentacyclic triterpenoid acids, suppress TNF-α-induced E-selectin expression by cultured umbilical vein endothelial cells. Phytomedicine 2010, 17, 1114–1119. [Google Scholar] [CrossRef]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol in Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Slamenová, D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat. Res. 2006, 600, 131–137. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Tufts, M.A.; Mapanga, R.F. Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 832–839. [Google Scholar] [CrossRef]
- Furtado, R.A.; Rodrigues, E.P.; Araújo, F.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon. Toxicol. Pathol. 2008, 36, 576–580. [Google Scholar] [CrossRef]
- Pott, P. Chirurgical Observations Relative to the Cancer of the Scrotum; Hawes, Clark, and Collins: London, UK, 1975; pp. 110–116. [Google Scholar]
- Poirier, M.C.; Beland, F.A. Aromatic amine-DNA adduct formation in chronically exposed mice: considerations for human comparison. Mutat. Res. 1997, 376, 177–184. [Google Scholar] [CrossRef]
- Weston, A.; Harris, C.C. Chemical Carcinogenesis. In Holland-FreiCancer Medicine, 5th ed.; Bast, R.C., Jr., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Holland, J.F., Frei, E., Eds.; BC Deker Inc.: Hamilton, ON, USA, 2000; pp. 193–198. [Google Scholar]
- Cerga (Vlaston), O.; Borcan, F.; Bernad, E.; Popovici, I. In vivo evaluation of cyclodextrin complexes with oleanolic and ursolic acids. J. Agroaliment. Process. Technol. 2012, 18, 130–135. [Google Scholar]
- Wang, H.M.; Soica, C.M.; Wenz, G. A comparison investigation on the solubilization of betulin and betulinic acid in cyclodextrin derivatives. Nat. Prod. Comm. 2012, 7, 289–291. [Google Scholar]
- Cerga, O.; Borcan, F.; Ambrus, R.; Popovici, I. Syntheses of new cyclodextrin complexes with oleanolic and ursolic acids. J. Agroaliment. Process. Technol. 2011, 17, 405–409. [Google Scholar]
- Sass, C.; Bojin, F.; Heges, A.; Galuscan, A.; Paunescu, V. Oleanolic and Ursolic Acid in Human Skin Cancer—A Preliminary in vitro comparative study. Fiziol. Physiol. 2012, 22, 30–33. [Google Scholar]
- Cerga, O.; Borcan, F.; Sass, C.; Galuscan, A.; Popovici, I. In Vitro Activity Of Ursolic and Oleanolic Acid On A2058 (Human Melanoma) And A2780 (Hepatic Carcinoma). Med. Evol. 2012, XVIII, 196–201. [Google Scholar]
- Andersson, D.; Liu, J.J.; Nilsson, A.; Duan, R.D. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res. 2003, 23, 3317–3322. [Google Scholar]
- Es-saady, D.; Simon, A.; Ollier, M.; Maurizis, J.C.; Chulia, A.J.; Delage, C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996, 106, 193–197. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Dingbo, S.; Cheng, J.; et al. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS One 2013, 8, e63872. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutierrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic Acid and Ursolic Acid Induce Apoptosis in HuH7 Human Hepatocellular Carcinoma Cells through a Mitochondrial-Dependent Pathway and Downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Li, H.; He, N.; Li, X.; Zhou, L.; Zhao, M.; Jiang, H.; Zhang, X. Oleanolic acid inhibits proliferation and induces apoptosis in NB4 cells by targeting PML/RARα. Oncol. Lett. 2013, 6, 885–890. [Google Scholar]
- Shan, J.; Xuan, Y.; Ruan, S.; Sun, M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin. J. Integr. Med. 2011, 17, 607–611. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Kannappan, R.; Ravindran, J.; Chaturvedi, M.M.; Vaahtera, L.; Parkkinen, J.; Aggarwal, B.B. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem. Pharmacol. 2010, 80, 1021–1032. [Google Scholar] [CrossRef]
- Mendonça, E.A.; Lira, M.C.; Rabello, M.M.; Cavalcanti, I.M.; Galdino, S.L.; Pitta, I.R.; Lima Mdo, C.; Pitta, M.G.; Hernandes, M.Z.; Santos-Magalhães, N.S. Enhanced antiproliferative activity of the new anticancer candidate LPSF/AC04 in cyclodextrin inclusion complexes encapsulated into liposomes. AAPS PharmSciTech 2012, 13, 1355–1366. [Google Scholar] [CrossRef]
- Danciu, C.; Soica, C.; Oltean, M.; Avram, S.; Borcan, F.; Csanyi, E.; Ambrus, R.; Zupko, I.; Muntean, D.; Dehelean, C.A.; et al. Genistein in 1:1 inclusion complexes with ramified cyclodextrins: Theoretical, physicochemical and biological evaluation. Int. J. Mol. Sci. 2014, 15, 1962–1982. [Google Scholar] [CrossRef]
- Dreassi, E.; Zizzari, A.T.; Mori, M.; Filippi, I.; Belfiore, A.; Naldini, A.; Carraro, F.; Santucci, A.; Schenone, S.; Botta, M. 2-Hydroxypropyl-β-cyclodextrin strongly improves water solubility and anti-proliferative activity of pyrazolo[3,4-d]pyrimidines Src-Abl dual inhibitors. Eur. J. Med. Chem. 2010, 45, 5958–5964. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Wangoo, K.T.; Morris, D.L. Albendazole-cyclodextrin complex: Enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 2008, 28, 2775–2779. [Google Scholar]
- Soica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin Complex in γ-Cyclodextrin Derivatives: Properties and antineoplasic activities in in vitro and in vivo tumor models. Int. J. Mol. Sci. 2012, 13, 14992–15011. [Google Scholar] [CrossRef]
- Buriez, O.; Heldt, J.M.; Labbé, E.; Vessières, A.; Jaouen, G.; Amatore, C. Reactivity and antiproliferative activity of ferrocenyl-tamoxifen adducts with cyclodextrins against hormone-independent breast-cancer cell lines. Chemistry 2008, 14, 8195–8203. [Google Scholar] [CrossRef]
- Hipler, U.C.; Schönfelder, U.; Hipler, C.; Elsner, P. Influence of cyclodextrins on the proliferation of HaCaT keratinocytes in vitro. J. Biomed. Mater. Res. Part A 2007, 83, 70–79. [Google Scholar]
- Ciurlea, S.; Bojin, M.F.; Csanyi, E.; Ionescu, I.; Borcan, F.; Galuscan, A.; Dehelean, C.A. Evaluation of skin parameters changes in chemical and photochemical initiated tumors on SKH1 mice. Fiziol. Physiol. 2011, 21, 18–22. [Google Scholar]
- McNeil, E.M.; Ritchie, A.M.; Melton, D.W. The toxicity of nitrofuran compounds on melanoma and neuroblastoma cells is enhanced by Olaparib and ameliorated by melanin pigment. DNA Repair (Amst) 2013, 12, 1000–1006. [Google Scholar] [CrossRef]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef]
- Liu, J.; Man, W.Y.; Lv, C.Z.; Song, S.P.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol. Physiol. 2010, 23, 193–200. [Google Scholar] [CrossRef]
- Gunathilake, R.; Schurer, N.Y.; Shoo, B.A.; Celli, A.; Hachem, J.P.; Crumrine, D.; Sirimanna, G.; Feingold, K.R.; Mauro, T.M.; Elias, P.M. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J. Invest. Dermatol. 2009, 129, 1719–1729. [Google Scholar] [CrossRef]
- Hachem, J.P.; Crumrine, D.; Fluhr, J.; Brown, B.E.; Feingold, K.R.; Elias, P.M. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J. Invest. Dermatol. 2003, 121, 345–353. [Google Scholar] [CrossRef]
- Tagami, H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), S1–S6. [Google Scholar]
- Seité, S.; Colige, A.; Piquemal-Vivenot, P.; Montastier, C.; Fourtanier, A.; Lapière, C.; Nusgens, B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2000, 16, 147–155. [Google Scholar]
- Scimeca, J.V.; Zimmerman, A.C.; Mettler, M.F.; Kudo, A.; Kawasaki, Y. Cosmetic Treatment System and Methods. U.S. Patent US7758878, 20 July 2010. B2. [Google Scholar]
- Farwick, M.; Köhler, T.; Schild, J.; Mentel, M.; Maczkiewitz, U.; Pagani, V.; Bonfigli, A.; Rigano, L.; Bureik, D.; Gauglitz, G.G. Pentacyclic triterpenes from Terminalia arjuna show multiple benefits on aged and dry skin. Skin Pharmacol. Physiol. 2014, 27, 71–81. [Google Scholar] [CrossRef]
- Hao, J.Q.; Li, Q.; Xu, S.P.; Shen, Y.X.; Sun, G.Y. Effect of lumiracoxib on proliferation and apoptosis of human nonsmall cell lung cancer cells in vitro. Chin. Med. J. (Engl.) 2008, 121, 602–607. [Google Scholar]
- Zhou, X.; Zhang, Y.; Li, Y.; Hao, X.; Liu, X.; Wang, Y. Azithromycin Synergistically Enhances Anti-Proliferative Activity of Vincristine in Cervical and Gastric Cancer Cells. Cancers (Basel) 2012, 4, 1318–1332. [Google Scholar] [CrossRef]
- CyclodextrinKnowledgeBase. Available online: http://www.cyclodextrin.net (accessed on 2 December 2013).
- Dehelean, C.A.; Feflea, S.; Gheorgheosu, D.; Ganta, S.; Cimpean, A.M.; Muntean, D.; Amiji, M.M. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in balb/c mice. J. Biomed. Nanotechnol. 2013, 9, 577–589. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the active compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.A.; Munteanu, M. The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin. Molecules 2014, 19, 4924-4940. https://doi.org/10.3390/molecules19044924
Soica C, Oprean C, Borcan F, Danciu C, Trandafirescu C, Coricovac D, Crăiniceanu Z, Dehelean CA, Munteanu M. The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin. Molecules. 2014; 19(4):4924-4940. https://doi.org/10.3390/molecules19044924
Chicago/Turabian StyleSoica, Codruţa, Camelia Oprean, Florin Borcan, Corina Danciu, Cristina Trandafirescu, Dorina Coricovac, Zorin Crăiniceanu, Cristina Adriana Dehelean, and Melania Munteanu. 2014. "The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin" Molecules 19, no. 4: 4924-4940. https://doi.org/10.3390/molecules19044924



