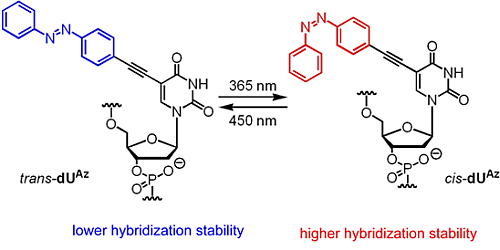
C5-Azobenzene-substituted 2'-Deoxyuridine-containing Oligodeoxynucleotides for Photo-Switching Hybridization
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of dUAz Phosphoramidite and dUAz-Modified Oligodeoxynucleotides

| ON | Sequence | |
|---|---|---|
| 6 | 5'-d(GCGTTTTTTGCT)-3' | control DNA |
| 7 | 5'-d(GCGTTUAzTTTGCT)-3' | dUAz-modified DNA |
| 8 | 5'-d(AGCAAAAAACGC)-3' | full match DNA |
| 9 | 5'-d(AGCAAATAACGC)-3' | mismatch DNA (T) |
| 10 | 5'-d(AGCAAACAACGC)-3' | mismatch DNA (C) |
| 11 | 5'-d(AGCAAAGAACGC)-3' | mismatch DNA (G) |
| 12 | 5'-r(AGCAAAAAACGC)-3' | full match RNA |
| 13 | 5'-r(AGCAAAUAACGC)-3' | mismatch RNA (U) |
| 14 | 5'-r(AGCAAACAACGC)-3' | mismatch RNA (C) |
| 15 | 5'-r(AGCAAAGAACGC)-3' | mismatch RNA (G) |
2.2. Photoisomerization Property of dUAz

| Duplex | Tm [°C] | ΔTm [°C] b (Tm cis - Tm trans) | ||
|---|---|---|---|---|
| trans c | cis d | |||
| 6/8 | 52 | - | ||
| 7/8 | 47 | 49 | 2 | |
| 6/12 | 47 | |||
| 7/12 | 42 | 47 | 5 | |
| Duplex | Base pair | Tm [°C] | ΔTm [°C] b | ||
|---|---|---|---|---|---|
| trans c | cis d | trans c | cis d | ||
| 6/9 | T:T | 40 | −12 | ||
| 6/10 | T:C | 37 | −15 | ||
| 6/11 | T:G | 41 | −11 | ||
| 7/9 | UAz:T | 33 | 35 | −14 | −14 |
| 7/10 | UAz:C | 33 | 34 | −14 | −15 |
| 7/11 | UAz:G | 33 | 35 | −14 | −14 |
3. Experimental
3.1. General
3.2. Preparation of 5-(4-Phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (1)
 −3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.
−3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.3.3. Preparation of 5'-O-(4,4'-Dimethoxytrityl)-5-(4-phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (4)
 36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.
36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.3.4. Preparation of 3-O-{2-Cyanoethyl(diisopropylamino)phosphino}-5'-O-(4,4'-Dimethoxytrityl)-5-(4-phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (5)
 32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.
32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.3.5. Synthesis of dUAz-Modified Oligodeoxynucleotides
| Oligodeoxynucleotide | Yield | MALDI-TOF MS | ||
|---|---|---|---|---|
| Calcd. [M-H]− | found [M-H]− | |||
| 5'-d(GCGTTUAzTTTGCT)-3' | 7 | 29% | 3822.6 | 3822.4 |
3.6. UV Melting Experiments
3.7. Photoisomerization of dUAz
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuzuya, A.; Komiyama, M. DNA origami: Fold, stick, and beyond. Nanoscale 2010, 2, 309–321. [Google Scholar]
- Torring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of comples structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef]
- Pinheiro, A.V.; Han, D.; Shin, W.M.; Yan, H. Challenges and opportunities for structural DNA nanotechnology. Nat. Nanotechnol. 2011, 6, 763–772. [Google Scholar] [CrossRef]
- Asanuma, H.; Ito, T.; Yoshida, T.; Liang, X.; Komiyama, M. Photoregulation of the formation and dissociation of a DNA duplex by using the cis-trans isomerization of azobenzene. Angew. Chem. Int. Ed. 1999, 38, 2393–2395. [Google Scholar] [CrossRef]
- Asanuma, H.; Liang, X.; Yoshida, T.; Komiyama, M. Photocontrol of DNA duplex formation by using azobenzene-bearing oligonucleotides. ChemBioChem 2001, 2, 39–44. [Google Scholar]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Dhammika, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar]
- Barrois, S.; Wagenknecht, H.A. Diarylehtene-modified nucleotides for switching optical properties inDNA. Beilstein. J. Org. Chem. 2012, 8, 905–914. [Google Scholar] [CrossRef]
- Xiao, Q.; Ranasinghe, R.T.; Tang, A.M.P.; Brown, T. Naphthalenyl- and anthracenyl-ethynyl dT analogues as base discriminating fluorescent nucleosides and intramolecular energy transfer donors in oligonucleotide probes. Tetrahedron 2007, 63, 3483–3490. [Google Scholar] [CrossRef]
- Franklin, R.E.; Gosling, R.G. Molecular configuration in sodium thymonucleate. Nature 1953, 171, 740–741. [Google Scholar] [CrossRef]
- Anderson, C.F.; Record, M.T., Jr. Salt-nucleic acid interactions. Annu. Rev. Phys. Chem. 1995, 46, 657–700. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Shirai, Y.; Sasaki, T; Guerrero, J.M.; Yu, B.; Hodge, P.; Tour, J.M. Synthesis and photoisomerization of Fullerene- and oligo(phenylene ethynylene)-azobenzene derivatives. ACS Nano 2008, 2, 97–106. [Google Scholar] [CrossRef]
- Matharu, A.S.; Jeeva, S.; Ramanujam, P.S. Liquid crystals for holographic optical data storage. Chem. Soc. Rev. 2007, 36, 1868–1880. [Google Scholar] [CrossRef]
- Liang, X.; Asanuma, H.; Komiyama, M. Photoregulation of DNA triplex formation by azobenzene. J. Am. Chem. Soc. 2002, 124, 1877–1883. [Google Scholar] [CrossRef]
- Nishioka, H.; Liang, X.; Asanuma, H. Effect of the ortho modification of azobenzene on the photoregulatory efficiency of DNA hybridization and the thermal stability of its cis form. Chem. Eur. J. 2010, 16, 2054–2062. [Google Scholar] [CrossRef]
- Asanuma, H.; Yoshida, T.; Ito, T.; Komiyama, M. Photo-responsive oligonucleotides carrying azobenzene at the 2'-position of uridine. Tetrahedron Lett. 1999, 40, 7995–7998. [Google Scholar] [CrossRef]
- Patnaik, S.; Kumar, P.; Garg, B.S.; Gandni, R.P.; Gupta, K.C. Photomodulation of PS-modified oligonucleotides containing azobenzene substituent at pre-selected positions in phosphate backbone. Bioorg. Med. Chem. 2007, 15, 7840–7849. [Google Scholar] [CrossRef]
- Ogasawara, S.; Maeda, M. Straightforward and reversible photoregulation of hybridization by using a photochromic nucleoside. Angew. Chem. Int. Ed. 2008, 47, 8839–8842. [Google Scholar] [CrossRef]
- Nishioka, H.; Liang, X.; Kashida, H.; Asanuma, H. 2',6'-Dimethylazobenzene as an efficient and thermo-stable photoregulator for the photoregulation of DNA hybridization. Chem. Commun. 2007, 4354–4356. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mori, S.; Morihiro, K.; Obika, S. C5-Azobenzene-substituted 2'-Deoxyuridine-containing Oligodeoxynucleotides for Photo-Switching Hybridization. Molecules 2014, 19, 5109-5118. https://doi.org/10.3390/molecules19045109
Mori S, Morihiro K, Obika S. C5-Azobenzene-substituted 2'-Deoxyuridine-containing Oligodeoxynucleotides for Photo-Switching Hybridization. Molecules. 2014; 19(4):5109-5118. https://doi.org/10.3390/molecules19045109
Chicago/Turabian StyleMori, Shohei, Kunihiko Morihiro, and Satoshi Obika. 2014. "C5-Azobenzene-substituted 2'-Deoxyuridine-containing Oligodeoxynucleotides for Photo-Switching Hybridization" Molecules 19, no. 4: 5109-5118. https://doi.org/10.3390/molecules19045109