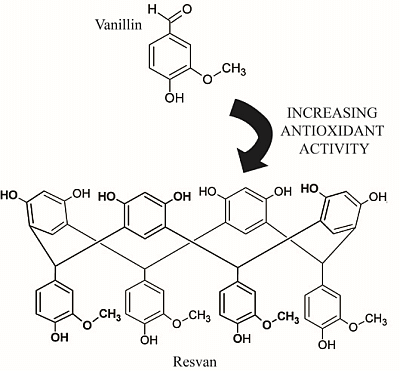
Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative
Abstract
:1. Introduction



2. Results and Discussion
2.1. Infrared Analysis
| Assignment | Resorcinol | Vanillin | Resvan |
|---|---|---|---|
| νO-H (phenol) | 3447 | 3447 | 3411 |
| νC-H (aromatic) | 3012 | 3013 | 3008 |
| νC-H (aldehyde) | 2855 | ||
| νC=O (aldehyde) | 1666 | ||
| νC-C (benzene) | 1616 | 1596 | 1616 and 1518 |
| νCH3 (methoxy) | 1463 | 1463 | |
| δCH (methine bridge) | 1430 | ||
| νC-CHO (aldehyde) | 1265 | ||
| νC-O (ether) | 1200 | 1204 | |
| δCH3 (methoxy) | 1172 | ||
| νC-OH | 1150 | 1155 | 1154 |
| δCCH (in-plane) | 1123 | 1123 | |
| νO-CH3 | 1028 | 1030 | |
| δC-C-CHO (out-of-plane) | 732 | ||
| δC-C=O (in-plane) | 588 |
2.2. Electronic Spectra Analysis

2.3. Citotoxicity and Acute Toxicity

2.4. Antioxidant Activity

2.5. Determination of in Vivo Antiprotozoal Activity of the Compounds



3. Experimental
3.1. Chemicals
3.2. Synthesis of C-4-Hydroxy-3-Methoxyphenylcalix [4] Resorcinarene (Resvan)
3.3. Apparatus
3.4. Cytotoxicity Assay
3.5. Animals
3.6. Acute Toxicity
3.7. Scavenging Activity on 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radicals
3.8. Determination of in Vivo Antiprotozoal Activity of the Compounds
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Borges, A.S. Some aspects of protozoan infections in immunocompromised patients—a review. Mem. Inst. Oswaldo. Cruz. 2002, 97, 443–457. [Google Scholar] [CrossRef]
- Lang, C.; Grob, U.; Luder, C.G.K. Subversion of innate and adaptative immune responses by Toxoplasma. gondii. Parasitol. Res. 2007, 100, 191–203. [Google Scholar] [CrossRef]
- Degerli, K.; Kilimcioğlu, A.A.; Kurt, Ö.; Tamay, A.T.; Özbilgin, A. Efficacy of azithromycin in a murine toxoplasmosis model, employing a Toxoplasma gondii strain from Turkey. Acta Trop. 2003, 88, 45–50. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Suzuki, Y. Effects of Toxoplasma gondii infection on the brain. Schizophr. Bull. 2007, 33, 745–751. [Google Scholar] [CrossRef]
- Kaye, A. Toxoplasmosis: Diagnosis, treatment, and prevention in congenitally exposed infants. J. Pediatr. Health. Care. 2011, 25, 355–364. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Hogh, B.; Andersen, O.; Hansen, S.T.; Dalhoff, K.; Petersen, E. Treatment of infants with congenital toxoplasmosis: Tolerability plasma concentrations of sulfadiazine and pyrimethamine. Eur. J. Pediatr. 2006, 165, 19–25. [Google Scholar] [CrossRef]
- Haverkos, H.W. Assessment of therapy for toxoplasma encephalitis: The TE study group. Am. J. Med. 1987, 82, 907–914. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef]
- Emami, S.A.; Asgary, S.; Naderi, G.A.; Ardekani, M.R.S.; Aslani, S.; Airin, A.; Kasher, T.; Sahebkar, A. Investigation of antioxidant and anti-glycation properties of essential oils from fruits and branchlets of Juniperus oblonga. Rev. Bras. Farmacogn. 2012, 22, 985–993. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Rasheed, S.; Nigam, P.S.; Janneh, O.; Sarker, S.D. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev. Bras. Farmacogn. 2011, 21, 943–952. [Google Scholar] [CrossRef]
- Moresco, H.H.; Queiroz, G.S.; Pizzolatti, M.G.; Brighente, I.M.C. Chemical constituents and evaluation of the toxic and antioxidant activities of Averrhoa carambola leaves. Rev. Bras. Farmacogn. 2012, 22, 319–324. [Google Scholar] [CrossRef]
- Kavitha, N.; Noordin, R.; Kit-Lam, C.; Sasidharan, S. Real Time Anti-Toxoplasma gondii Activity of an Active Fraction of Eurycoma. longifolia Root Studied by in Situ Scanning and Transmission Electron Microscopy. Molecules 2012, 17, 9207–9219. [Google Scholar] [CrossRef]
- Zhai, B.; Clark, J.; Ling, T.; Connelly, M.; Medina-Bolivar, F.; Rivas, F. Antimalarial Evaluation of the Chemical Constituents of Hairy Root Culture of Bixa. orellana L. Molecules 2014, 19, 756–766. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). The World Health Report 1999: Making a difference; WHO: Geneva, Switzerland, 1999; pp. 31–63. [Google Scholar]
- Oliveira, F.Q.; Andrade-Neto, V.; Krettli, A.U.; Brandão, M.G.L. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 2004, 93, 39–42. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. BBA-Gen. Subjects. 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Narbad, A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food. Microbiol. 2003, 86, 113–122. [Google Scholar] [CrossRef]
- Vaghasiya, Y.K.; Nair, R.; Soni, M.; Baluja, S.; Chanda, S. Synthesis, structural determination and antibacterial activity in compounds derived from vanillin and 4-aminoantipyrine. J. Serb. Chem. Soc. 2004, 69, 991–998. [Google Scholar] [CrossRef]
- Rakchoy, S.; Suppakul, P.; Jinkarn, T. Antimicrobial effects of vanillin coated solution for coating paperboard intended for packaging bakery products. As. J. Food. Ag-Ind. 2009, 2, 138–147. [Google Scholar]
- Imanishi, H.; Sasaki, Y.; Matsumoto, K.; Watanabe, M.; Ohta, T.; Shirasu, Y.; Tutikawa, K. Suppression of 6-TG-resistant mutations in V79 cells and recessive spot formations in mice by vanillin. Mutat. Res. Lett. 1990, 243, 151–158. [Google Scholar] [CrossRef]
- Ohta, T.; Watanabe, M.; Watanabe, K.; Shirasu, Y.; Kada, T. Inhibitory effects of flavourings on mutagenesis induced by chemicals in bacteria. Food Chem. Toxicol. 1986, 24, 51–54. [Google Scholar] [CrossRef]
- Ho, K.; Yazan, L.S.; Ismail, N.; Ismail, M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Iwaki, A.; Ohya, Y.; Izawa, S. Vanillin causes the activation of Yap1 and mitochondrial fragmentation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2014, 117, 33–38. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food. Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Beaudry, F.; Ross, A.; Lema, P.P.; Vachon, P. Pharmacokinetics of vanillin and its effects on mechanical hypersensitivity in a rat model of neuropathic pain. Phytother. Res. 2010, 24, 525–530. [Google Scholar]
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.A.; Cavaleiro, C.; Sousa, M.C. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef]
- Ito, H.; Nakayama, T.; Sherwood, M.; Miller, D.; Ueda, M. Characterization and Lithographic Application of Calix[4]resorcinarene Derivatives. Chem. Mater. 2008, 20, 341–356. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ponnusamy, S. Vibrational spectra and normal coordinate analysis on an organic non-linear optical crystal-3-methoxy-4-hydroxy benzaldehyde. Indian. J. Pure. Ap. Phy. 2005, 43, 838–843. [Google Scholar]
- Alonso Moreno, Y.; Trapero Quintana, Y.M. Toxicidad aguda oral de la o-vainillina. Rev. Cubana. Farm. 2008, 42, 0–0. [Google Scholar]
- (OECD) Organization for Economic Cooperation and Development. Guidelines 423, Acute Oral Toxicity-Acute Toxic Class Method. In OECD Guidelines for Testing of Chemicals; OECD: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the malaysian rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food. Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, D.; Sheng, J.; Li, Z.; Qi, H.; Sun, Y.; Duan, Y.; Zhang, W. Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 2013, 56, 1–5. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohyd. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Barla, A.; Ozturk, M.; Kultur, S.; Oksuz, S. Screening of antioxidant activity of three Euphorbia species from Turkey. Fitoterapia 2007, 78, 423–425. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef]
- Azzi, A.; Davies, K.J.; Kelly, F. Free radical biology - terminology and critical thinking. FEBS Lett. 2004, 558, 3–6. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, E.M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Silva, D.A.O.; Rostkowska, C.; Béla, S.R.; Ferro, E.A.V.; Magalhães, P.M.; Mineo, J.R. Toxoplasma gondii: Effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp. Parasitol. 2009, 122, 233–241. [Google Scholar] [CrossRef]
- Silva, T.A.C.; Chioccola, V.L.P. Toxoplasma gondii acute infection: Estimation of humoral response and blood parasitism in mice AS/n inbred. Sci. Med. 2010, 20, 88–92. [Google Scholar]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradizoytes, and sporozoytes and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar]
- Sullivan, W.J.; Jeffers, V. Mechanisms of Toxoplasma Gondii persistence and latency. FEMS Microbiol. Rev. 2012, 36, 717–733. [Google Scholar] [CrossRef]
- McCarthy, S.M.; Davis, C.D. Prooxidant diet provides protection during murine infection with Toxoplasma gondii. J. Parasitol. 2003, 89, 886–894. [Google Scholar] [CrossRef]
- Rose, K.N.; Hardie, M.J.; Atwood, J.L.; Raston, C.L. Oxygen-center laden C2h symmetry resorcin[4]arenes. J. Supramol. Chem. 2001, 1, 35–38. [Google Scholar] [CrossRef]
- Tempone, A.G.; Treiger Borborema, S.E.; Andrade, H.F., Jr.; Amorim Gualda, N.C.; Yogi, A.; Salerno Carvalho, C.; Bachiega, D.; Lupo, F.N.; Bonotto, S.V.; Fischer, D.C.H. Antiprotozoal activity of Brazilian plant extracts from isoquinoline alkaloid-producing families. Phytomedicine 2005, 12, 382–390. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oliveira, C.B.S.; Meurer, Y.S.R.; Oliveira, M.G.; Medeiros, W.M.T.Q.; Silva, F.O.N.; Brito, A.C.F.; Pontes, D.D.L.; Andrade-Neto, V.F. Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative. Molecules 2014, 19, 5898-5912. https://doi.org/10.3390/molecules19055898
Oliveira CBS, Meurer YSR, Oliveira MG, Medeiros WMTQ, Silva FON, Brito ACF, Pontes DDL, Andrade-Neto VF. Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative. Molecules. 2014; 19(5):5898-5912. https://doi.org/10.3390/molecules19055898
Chicago/Turabian StyleOliveira, Claudio B. S., Ywlliane S. R. Meurer, Marianne G. Oliveira, Wendy M. T. Q. Medeiros, Francisco O. N. Silva, Ana C. F. Brito, Daniel De L. Pontes, and Valter F. Andrade-Neto. 2014. "Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanillin and Its Resorcinarene Derivative" Molecules 19, no. 5: 5898-5912. https://doi.org/10.3390/molecules19055898