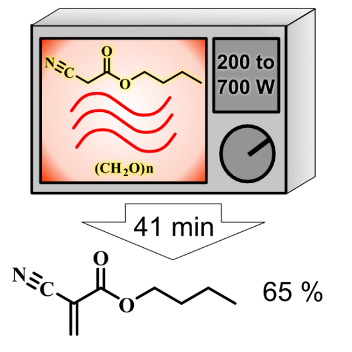
n-Butyl Cyanoacrylate Synthesis. A New Quality Step Using Microwaves
Abstract
:1. Introduction
2. Results and Discussion



| Heating Source | Exp. | Time (min) | Total Time (min) | Monomer Yield (%) | |||
|---|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 3th Stage | 4th Stage | ||||
| Oil bath | 1 | 40 | 36 | 60 | 23 | 159 | 72 |
| 2 | 48 | 17 | 57 | 17 | 139 | 63 | |
| 3 | 40 | 43 | 87 | 20 | 190 | 67 | |
| Mean values | 163 ± 26 | 67 ± 5 | |||||
| Microwave oven | 1 | 24 | - | 15 | - | 39 | 72 |
| 2 | 9 | - | 38 | - | 47 | 63 | |
| 3 | 10 | - | 27 | - | 37 | 61 | |
| Mean values | 41 ± 5 | 65 ± 6 | |||||


| Heating Source | Exp. | Time (min) | Yield (%) |
|---|---|---|---|
| Oil bath | 1 | 75 | 81 |
| 2 | 58 | 76 | |
| 3 | 65 | 65 | |
| Mean values | 66 ± 9 | 74 ± 8 | |
| Microwave oven | 1 | 40 | 85 |
| 2 | 37 | 72 | |
| 3 | 51 | 50 | |
| Mean values | 43 ± 7 | 69 ± 18 |
3. Experimental Section
3.1. General
3.2. Direct Synthesis of BCA Using an Oil Bath
3.3. Direct Synthesis of BCA Assisted by Microwave
3.4. Indirect Synthesis of BCA Using an Oil Bath and a Microwave Oven
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anonymous. Super Glue. Available online: http://web.mit.edu/invent/iow/coover.html (accessed on 22 June 2010).
- Dadas, B.; Alkan, S.; Cifci, M.; Basak, T. Treatment of tripod fracture of zygomatic bone by n-2-butyl cyanoacrylate glue fixation, and its effects on the tissues. Eur. Arch. Otorhinolaryngol. 2007, 264, 539–544. [Google Scholar] [CrossRef]
- Voon, L.W.; Chua, C.N.; Hanson, R. The use of n-butyl cyanoacrylate (indermil) in lateral tarsorrhaphy. Arch. Ophthalmol. 2004, 122, 279–281. [Google Scholar] [CrossRef]
- Cantasdemir, M.; Adaletli, I.; Cebi, D.; Kantarci, F.; Selcuk, N.D.; Numan, F. Emergency endovascular embolization of traumatic intrarenal arterial pseudoaneurysms with n-butyl cyanoacrylate. Clin. Radiol. 2003, 58, 560–565. [Google Scholar] [CrossRef]
- Lo, G.-H.; Lai, K.-H.; Cheng, J.-S.; Chen, M.-H.; Chiang, H.-T. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology 2001, 33, 1060–1064. [Google Scholar] [CrossRef]
- Grisdale, J. The use of cyanoacrylates in periodontal therapy. J. Can. Dent. Assoc. 1998, 64, 632–633. [Google Scholar]
- Romero, I.L.; Malta, J.B.N.S.; Silva, C.B.; Mimica, L.M.J.; Soong, K.H.; Hida, R.Y. Antibacterial properties of cyanoacrylate tissue adhesive: Does the polymerization reaction play a role? Indian J. Ophthalmol. 2009, 57, 341–344. [Google Scholar] [CrossRef]
- Jee, Y.; Ho, J.; Jong, H.; Min, B.; Chan, J.; Ki, S.; Yong, H.; Sung, M. Endoscopic hemostasis using n-butyl-2-cyanoacrylate for massive gastric bleeding durng endoscopic transgastric drainage of pancreatic pseudocyst. Dig. Endosc. 2011, 23, 326–327. [Google Scholar] [CrossRef]
- Reukov, V.; Maximov, V.; Vertegel, A. Proteins conjugated to poly(butyl cyanoacrylate) nanoparticles as potential neuroprotective agents. Biotechnol. Bioeng. 2010, 108, 243–252. [Google Scholar] [CrossRef]
- Joshi, S.A.; Chavhan, S.S.; Sawant, K.K. Rivastigmine-loaded plga and pbca nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur. J. Pharm. Biopharm. 2010, 76, 189–199. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lee, Y.D. Core-shell type of nanoparticles composed of poly((n-butyl cyanoacrylate)-co-(2-octyl cyanoacrylate)) copolymers for drug delivery application: Synthesis, characterization and in vitro degradation. Int. J. Pharm. 2006, 325, 132–139. [Google Scholar] [CrossRef]
- Ardis, A.E.; Akron, O. Preparation of Monomeric Alkyl α-cyanoacrylates. U.S. Patent 2,467,926, 19 April 1949. [Google Scholar]
- Meier, E.A.; Ray-Chaudhuri, D.K.; Schoenberg, J.E. Process for the Manufacture of Methyl Cyanoacrylate. U.S. Patent 5,455,369, 3 October 1995. [Google Scholar]
- Sailhan, V.; Schue, F.; Eloy, R.; Giral, L. Highly Pure Alkyl 2-cyanoacrylates. EP 0 895 987; B1, 31 March 2004. [Google Scholar]
- Jain, S.; Nagi Reddy, B.; Sambasiva Rao, K.; Neeliah, G. Microwave assisted synthesis of indole substituted alkenes using knoevenagel condensation reaction and their antibacterial activity study. E-J. Chem. 2010, 7, 543–551. [Google Scholar] [CrossRef]
- Mallouk, S.; Bougrin, K.; Laghzizil, A.; Benhida, R. Microwave-assisted and efficient solvent-free knoevenagel condensation. A sustainable protocol using porous calcium hydroxyapatite as catalyst. Molecules 2010, 15, 813–823. [Google Scholar] [CrossRef]
- Mogilaiah, K.; Prashanthi, M.; Reddy, G.R.; Reddy, C.S.; Reddy, N.V. Microwave assisted knoevenagel condensation using sodium fluoride and lithium chloride as catalysts under solvent-free conditions. Synth. Commun. 2003, 33, 2309–2312. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent free organic synthesis using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Balalaie, S.; Nemati, N. Ammonium acetate-basic alumina catalyzed knoevenagel condensation under microwave irradiation under solvent-free condition. Synth. Commun. 2000, 30, 869–875. [Google Scholar] [CrossRef]
- Mitra, A.K.; De, A.; Karchaudhurin, N. Solvent-free microwave enhanced knoevenagel condensation of ethyl cyanoacetate with aldehydes. Synth. Commun. 1999, 29, 2731–2739. [Google Scholar] [CrossRef]
- Chorbadjiev, K.G.; Novakov, P.C. Study of the thermal degradation of poly(alkyl α-cyanoacrylate)s. Eur. Polym. J. 1991, 27, 1009–1015. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds n-butyl cyanoacrylate and poly (n-butyl-cyanoacrylate) are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carriles, Y.R.; Brito, R.Á.; Sánchez, R.M.; Acevedo, E.S.; Domínguez, P.R.; Mueller, W.-D. n-Butyl Cyanoacrylate Synthesis. A New Quality Step Using Microwaves. Molecules 2014, 19, 6220-6227. https://doi.org/10.3390/molecules19056220
Carriles YR, Brito RÁ, Sánchez RM, Acevedo ES, Domínguez PR, Mueller W-D. n-Butyl Cyanoacrylate Synthesis. A New Quality Step Using Microwaves. Molecules. 2014; 19(5):6220-6227. https://doi.org/10.3390/molecules19056220
Chicago/Turabian StyleCarriles, Yaquelin Ramos, Rubén Álvarez Brito, Ricardo Martínez Sánchez, Elayma Sánchez Acevedo, Paola Rodríguez Domínguez, and Wolf-Dieter Mueller. 2014. "n-Butyl Cyanoacrylate Synthesis. A New Quality Step Using Microwaves" Molecules 19, no. 5: 6220-6227. https://doi.org/10.3390/molecules19056220