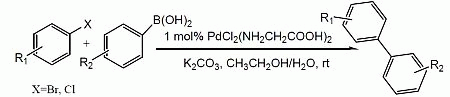A Simple Hydrophilic Palladium(II) Complex as a Highly Efficient Catalyst for Room Temperature Aerobic Suzuki Coupling Reactions in Aqueous Media
Abstract
:1. Introduction
2. Results and Discussion


| Entry | Solvent | Base | Yield b (%) |
|---|---|---|---|
| 1 | Water | Na2CO3 | 39 |
| 2 | Water | K2CO3 | 49 |
| 3 | Water | Na3PO4 | 26 |
| 4 | Water | NaOAc | 30 |
| 5 | Water | Cs2CO3 | 35 |
| 6 | Water | NaF | 43 |
| 7 | Water | NaOH | 40 |
| 8 c | Methanol + Water | K2CO3 | 24 |
| 9 c | Ethanol + Water | K2CO3 | 99 |
| 10 c | Propanol + Water | K2CO3 | 76 |
| 11 c | Isopropanol + Water | K2CO3 | 85 |
| 12 c | N-Butyl alcohol + Water | K2CO3 | 18 |
| Entry | Aryl halide | Arylboronic acid | Product | Yield b (%) | |||
|---|---|---|---|---|---|---|---|
| 1 |  |  |  | 94 | |||
| 2 |  |  |  | 90 | |||
| 3 |  |  |  | 99 | |||
| 4 |  |  |  | 95 | |||
| 5 |  |  |  | 86 | |||
| 6 |  |  |  | 94 | |||
| 7 |  |  |  | 99 | |||
| 8 |  |  |  | 88 | |||
| 9 |  |  |  | 32 | |||
| 10 |  |  |  | 68 | |||
| 11 |  |  |  | 64 | |||
| 12 c |  |  |  | 77 | |||
| 13 c |  |  |  | 73 | |||
| 14 c |  |  |  | 69 | |||
| 15 c |  |  |  | 66 | |||
3. Experimental
3.1. General Information
3.2. General Procedure for the Preparation of the Hydrophilic Palladium(II) Complex 2
3.3. General Procedure for the Suzuki Type Coupling Reactions
3.4. Analytical Data of Representative Products
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Miyaura, N. Organoboron compounds. Top. Curr. Chem. 2002, 219, 11–59. [Google Scholar] [CrossRef]
- Molnar, A. Efficient, selective and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C-C bonds (nobel lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Old, D.W.; Wolfe, J.P.; Buchwald, S.L. A highly active catalyst for palladium-catalyzed cross-coupling reactions: Room-temperature suzuki couplings and amination of unactivated aryl chlorides. J. Am. Chem. Soc. 1998, 120, 9722–9723. [Google Scholar] [CrossRef]
- Littke, A.F.; Dai, C.; Fu, G.C. Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions. J. Am. Chem. Soc. 2000, 122, 4020–4028. [Google Scholar] [CrossRef]
- Dai, W.; Li, Y.; Zhang, Y.; Yue, C.; Wu, J. Generation of an aromatic amide derived phosphane (aphos) library by self-assisted molecular editing and applications of aphos in room-temperature Suzuki-miyaura reactions. Chem. Eur. J. 2008, 14, 5538–5554. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Taft, B.R.; Abela, A.R.; Ghorai, S.; Krasovskiy, A. Catalysis in the service of green chemistry: Nobel prize-winning palladium-catalysed cross-couplings, run in water at room temperature. Platinum Metals Rev. 2012, 56, 62–74. [Google Scholar]
- Banik, B.; Tairai, A.; Shahnaz, N.; Das, P. Palladium(II) Complex with a Potential N4-Type Schiff-Base Ligand as Highly Efficient Catalyst for Suzuki-Miyaura Reactions in Aqueous Media. Tetrahedron Lett. 2012, 53, 5627–5630. [Google Scholar] [CrossRef]
- Modak, A.; Mondal, J.; Sasidharan, M.; Bhaumik, A. Triazine Functionalized Ordered Mesoporous Polymer: A Novel Solid Support for Pd-Mediated C-C Cross-Coupling Reactions in Water. Green Chem. 2011, 13, 1317–1331. [Google Scholar] [CrossRef]
- Herrerías, C.I.; Yao, X.; Li, Z.; Li, C. Reactions of C-H Bonds in Water. Chem. Rev. 2007, 107, 2546–2562. [Google Scholar] [CrossRef]
- Watson, W.J.W. How to the Fine Chemical, Pharmaceutical, and Related Industries Approach Green Chemistry and Sustainability? Green Chem. 2012, 14, 251–259. [Google Scholar]
- Casalnuovo, A.L.; Calabrese, J.C. Palladium-Catalyzed Alkylations in Aqueous Media. J. Am. Chem. Soc. 1990, 112, 4324–4330. [Google Scholar] [CrossRef]
- Shaughnessy, K.H. Hydrophilic Ligands and their Application in Aqueous-Phase Metal-Catalyzed Reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.X.; Niu, N.; Qiu, J.H. A simple and efficient approach for the palladium-catalyzed ligand-free Suzuki reaction in water. Green Chem. 2012, 14, 2999–3003. [Google Scholar] [CrossRef]
- Martín, L.; Molins, E.; Vallribera, A. Nickel and Palladium Nanocomposite Carbon Aerogels as Recyclable Catalysts for Suzuki-Miyaura Reaction under Aerobic and Phosphine-Free Conditions in Water. Tetrahedron 2012, 68, 6517–6520. [Google Scholar] [CrossRef]
- Mondal, P.; Banerjee, S.; Roy, A.S.; Mandal, T.K.; Islam, S.M. In situ Prepared Mesoporous Silica Nanosphere Supported Palladium(II) 2-aminopyridine Complex Catalyst for Suzuki-Miyaura Cross-Coupling Reaction in Water. J. Mater. Chem. 2012, 22, 20434–20442. [Google Scholar] [CrossRef]
- Zhou, P.P.; Wang, H.H.; Yang, J.Z.; Tang, J.; Sun, D.P.; Tang, W.H. Bio-supported Palladium Nanoparticles as a Phosphine-Free Catalyst for the Suzuki Reaction in Water. RSC Adv. 2012, 2, 1759–1761. [Google Scholar] [CrossRef]
- Kostas, I.D.; Coutsolelos, A.G.; Charalambidis, G.; Skondra, A. The First Use of Porphyrins as Catalysts in Cross-Coupling Reactions: a Water-Soluble Palladium Complex with a Porphyrin Ligand as an Efficient Catalyst Precursor Media under Aerobic Conditions. Tetrahedron Lett. 2007, 48, 6688–6691. [Google Scholar] [CrossRef]
- Gülcemal, S.; Kani, I.; Yilmaz, F.; Cetinkaya, B. N,N,N',N'-Tetrakis(2-hydroxyethyl)ethylene Diamine Palladium(II) Complex as Efficient Catalyst for the Suzuki/Miyaura Reaction in Water. Tetrahedron 2010, 66, 5602–5606. [Google Scholar] [CrossRef]
- Tu, T.; Feng, X.K.; Wang, Z.X. A Robust Hydrophilic Pyridine-Bridged Bis-benzimidazolylidene Palladium Pincer Complex: Synthesis and Its Catalytic Application towards Suzuki-Miyaura Coupling in Aqueous Solvents. Dalton Trans. 2010, 39, 10598–10600. [Google Scholar] [CrossRef]
- Bai, S.Q.; Hor, T.S.A. Isolation of an [SNS]Pd(II)pincer with a Water Ladder and Its Suzuki Coupling Activity in Water. Chem. Commun. 2008, 2008, 3172–3174. [Google Scholar]
- Beller, M.; Krauter, J.G.E.; Zapf, A. Carbohydrate-Substituted Triaryl Phosphanes—A New Class of Ligands for Two-Phase Catalysis. Angew. Chem. Int. Ed. 1997, 36, 772–774. [Google Scholar] [CrossRef]
- Anderson, K.W.; Buchwald, S.L. General Catalysts for the Suzuki-Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water. Angew. Chem. Int. Ed. 2005, 44, 6173–6177. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Jin, Z.L. Poly(ethylene glycol)-functionalized imidazolium salts-palladium-catalyzed Suzuki reaction in water. Green Chem. 2012, 14, 592–597. [Google Scholar] [CrossRef]
- Zhi, J.; Song, D.P.; Li, Z.W. Palladium Nanoparticles in Carbon Thin Film-Lined SBA-15 Nanoreactors: Efficient heterogeneous Catalysts for Suzuki-Miyaura Cross Coupling Reaction in Aqueous Media. Chem. Commun. 2011, 47, 10707–10709. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Abela, A.R.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A.; Gaston, R.D.; Gadwood, R.C. TPGS-750-M: A second-generation amphiphile for metal-catalyzed cross-coupling in water at room temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar]
- Krasovskiy, A.; Thomé, I.; Graff, J.; Krasovskaya, V.; Konopelski, P.; Duplais, C.; Lipshutz, B.H. Cross-Coupling of Alkyl Halides with Heteroaromatic Halides, in Water at Room Temperature. Tetrahedron Lett. 2011, 52, 2203–2205. [Google Scholar]
- Jin, M.J.; Lee, D.H. A Practical Heterogeneous Catalyst for the Suzuki, Sonogashira, and Stille Coupling Reactions of Unreactive Aryl Chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.S.; Wang, R.H. Palladium Nanoparticles Immobilized by Click Ionic Copolymers: Efficient and Recyclable Catalysts for Suzuki-Miyaura Cross-Coupling Reaction in Water. Catal. Commun. 2012, 22, 83–88. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. An efficient protocol for palladium-catalyzed ligand-free Suzuki-Miyaura coupling in water. Green Chem. 2012, 14, 1873–1876. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, L.; Liu, M.; Shen, Q.; Tang, J. An Efficient and Simple Protocol for PdCl2-Ligandless and Additive-Free Suzuki Coupling Reaction of Aryl Bromides. Tetrahedron Lett. 2011, 52, 6489–6491. [Google Scholar] [CrossRef]
- De souza, A.L.; da silva, F.L.C.; Oliveira, B.L.; Antunes, O.A.C. Microwave- and ultrasound-assisted Suzuki-Miyaura cross-coupling reactions catalyzed by Pd/PVP. Tetrahedron Lett. 2008, 49, 3895–3898. [Google Scholar]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Hanhan, M.E.; Martínez-Máñez, R.; Ros-Lis, J.V. Highly Effective Activation of Aryl Chlorides for Suzuki Coupling in Aqueous Media using a Ferrocene-Based Pd(II)-Diimine Catalyst. Tetrahedron Lett. 2012, 53, 2388–2391. [Google Scholar]
- Guo, M.M.; Jian, F.F.; He, R. Cross-Coupling Reactions Catalyzed by P, O Chelate Palladium Complexes at Room Temperature. Tetrahedron Lett. 2005, 46, 9017–9020. [Google Scholar] [CrossRef]
- Guo, M.M.; Jian, F.F.; He, R. The Air-Stable and Highly Efficient P, N-Chelated Palladium(II) Complexes as Catalysts for the Suzuki Cross-Coupling Reaction at Room Temperature. Tetrahedron Lett. 2006, 47, 2033–2036. [Google Scholar] [CrossRef]
- Guo, M.M.; Jian, F.F.; He, R. Efficient Synthesis of Fluorinated Biphenyl Derivatives via Pd-Catalyzed Suzuki Coupling Reaction in Aqueous Solvents at Room Temperature. J. Fluo. Chem. 2006, 127, 177–181. [Google Scholar] [CrossRef]
- Guo, M.M.; Zhang, Q.C. An Inexpensive and Highly Stable Palladium(II) Complex for Room Temperature Suzuki Coupling Reactions under Ambient Atmosphere. Tetrahedron Lett. 2009, 50, 1965–1968. [Google Scholar] [CrossRef]
- Guo, M.M.; Zhu, Z.Y.; Huang, H.W.; Zhang, Q.C. Phosphine-free Palladium Catalyzed Suzuki Coupling of Aryl Chlorides in Morpholine under Aerobic Conditions. Catal. Commun. 2009, 10, 865–867. [Google Scholar] [CrossRef]
- Tao, B.; Boykin, D.W. tran-Pd(OAc)2(Cy2NH)2 Catalyzed Suzuki Coupling Reactions and Its Temperature-Dependent Activities toward Aryl Bromides. Tetrahedron Lett. 2003, 44, 7993–7996. [Google Scholar]
- Pandiarajan, D.; Ramesh, R. Suzuki-Miyaura Cross-Coupling Reaction of Aryl Bromides Catalyzed by Palladium(II) Pyridoxal Hydrazone Complexes. J. Organomet. Chem. 2012, 708-709, 18–24. [Google Scholar] [CrossRef]
- Chu, W.Y.; Li, X.M.; Hou, Y.J.; Wang, H.; Li, H.Y.; Yuan, X.B.; Sun, Z.Z. 18-Crown-6 Promoting Pd/C-Catalyzed Cross-Coupling Reaction of Aryl Bromides and Arylboronic Acids in Aqueous Media. Appl. Organometal. Chem. 2012, 26, 478–482. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, Z.G.; Liu, Y.L.; Huang, L.; Yu, H.W.; Xie, Y.R.; Lai, C. A Highly Active and Stable Imidazolidine N,O-donor Ligand for Efficient Palladium Catalyzed Suzuki-Miyaura Reaction in Water. Transition met. Chem. 2012, 37, 331–335. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Cao, J.; Zhang, S. Guanidine/Pd(OAc)2-Catalyzed Room Temperature Suzuki Cross- Coupling Reaction in Aqueous Media under Aerobic Conditions. J. Org. Chem. 2007, 72, 4067–4072. [Google Scholar] [CrossRef]
- Roy, S.; Plenio, H. Sulfonated N-Heterocyclic Carbenes for Pd-Catalyzed Sonogashira and Suzuki-Miyaura Coupling in Aqueous Solvents. Adv. Synth. Catal. 2010, 352, 1014–1022. [Google Scholar] [CrossRef]
- Godoy, F.; Segarra, C.; Poyatos, M.; Peris, E. Palladium catalysts with sulfonate-functionalized-NHC ligands for Suzuki-Miyaura cross-coupling reaction in water. Organometallics 2011, 30, 684–688. [Google Scholar]
- Jarolìm, T.; Podlahová, J. Coordinating Behaviour of Diphenylphosphineacetic Acid. J. Inorg. Nucl. Chem. 1976, 38, 125–129. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds trans-PdCl2(NH2CH2COOH)2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, M.; Liu, S.; Zhou, X.; Lv, M.; Chen, S.; Xiao, D. A Simple Hydrophilic Palladium(II) Complex as a Highly Efficient Catalyst for Room Temperature Aerobic Suzuki Coupling Reactions in Aqueous Media. Molecules 2014, 19, 6524-6533. https://doi.org/10.3390/molecules19056524
Guo M, Liu S, Zhou X, Lv M, Chen S, Xiao D. A Simple Hydrophilic Palladium(II) Complex as a Highly Efficient Catalyst for Room Temperature Aerobic Suzuki Coupling Reactions in Aqueous Media. Molecules. 2014; 19(5):6524-6533. https://doi.org/10.3390/molecules19056524
Chicago/Turabian StyleGuo, Mengping, Shiwen Liu, Xiuling Zhou, Meiyun Lv, Sanbao Chen, and Daoan Xiao. 2014. "A Simple Hydrophilic Palladium(II) Complex as a Highly Efficient Catalyst for Room Temperature Aerobic Suzuki Coupling Reactions in Aqueous Media" Molecules 19, no. 5: 6524-6533. https://doi.org/10.3390/molecules19056524