A Novel Aqueous Two Phase System Composed of a Thermo-Separating Polymer and an Organic Solvent for Purification of Thermo-Acidic Amylase Enzyme from Red Pitaya (Hylocereus polyrhizus) Peel
Abstract
:1. Introduction
2. Result and Discussion
2.1. The Influence of Phase Components on the Amylase Activity
| Phase composition | Concentration (%) | Relative activity | |||
|---|---|---|---|---|---|
| EOPO2500 | 10 | 101.1 ± 1.33 a | |||
| 30 | 130.4 ± 0.21 | ||||
| 50 | 103.2 ± 0.01 e | ||||
| 70 | 100.2 ± 0.00 a | ||||
| EOPO 3900 | 10 | 99.3 ± 0.11 a | |||
| 30 | 97.1 ± 0.21 a | ||||
| 50 | 101.2 ± 0.32 d | ||||
| 70 | 98.6 ± 0.57 ab | ||||
| EOPO 12000 | 10 | 83.2 ± 0.09 f | |||
| 30 | 78.2 ± 1.42 g | ||||
| 50 | 66.3 ± 0.20 g | ||||
| 70 | 67.1 ± 0.32 h | ||||
| Ethanol | 10 | 97.2 ± 0.12 a | |||
| 30 | 88.1 ± 0.07 e | ||||
| 50 | 86.6 ± 0.10 e | ||||
| 70 | 83.2 ± 0.52 b | ||||
| 1-propanol | 10 | 100.2 ± 0.22 a | |||
| 30 | 126.1 ± 0.07 e | ||||
| 50 | 118.6 ± 1.10 e | ||||
| 70 | 98.2 ± 0.52 b | ||||
| 2-propanol | 10 | 140.2 ± 1.23 i | |||
| 30 | 115.2 ± 0.16 d | ||||
| 50 | 121.6 ± 0.02 e | ||||
| 70 | 101.6 ± 0.0 a | ||||
2.2. Optimization of EOPO/2-Propanol ATPS
2.3. Phase Diagram of EOPO Copolymer/Organic Solvent ATPS
| Mixing Point | Purification Factor | Yield (%) | |
|---|---|---|---|
| EOPO 2500 (%,w/w) | 2-Propanol (%,w/w) | ||
| 10 | 15 | 8.5 ± 0.12 a | 50.4 ± 0.15 a |
| 10 | 35 | 8.8 ± 1.32 a | 51.2 ± 0.08 a |
| 10 | 55 | 9.3 ± 0.81 b | 62.3 ± 0.04 b |
| 10 | 75 | 9.7 ± 0.52 b | 74.1 ± 0.12 c |
| 30 | 15 | 12.6 ± 0.01 c | 94.8 ± 0.13 d |
| 30 | 35 | 10.2 ± 0.11 d | 93.6 ± 0.01 e |
| 30 | 55 | 10.3 ± 0.05 c | 72.5 ± 0.02 c |
| 30 | 75 | 9.2 ± 0.14 b | 64.2 ± 0.10 b |
| 50 | 15 | 8.4 ± 0.08 a | 73.2 ± 0.02 c |
| 50 | 35 | 7.9 ± 0.13 e | 62.1 ± 0.11 b |
| 50 | 55 | 7.2 ± 0.14 e | 60.5 ± 0.12 b |
| 50 | 75 | 6.8 ± 0.21 e | 52.8 ± 0.22 a |
| 70 | 15 | 5.8 ± 0.07 f | 43.1 ± 0.01 f |
| 70 | 35 | 5.2 ± 0.01 f | 40.2 ± 0.22 f |
| 70 | 55 | 3.6 ± 0.17 h | 37.3 ± 0.09 g |
| 70 | 75 | 1.2 ± 0.04 i | 33.5 ± 0.06 g |
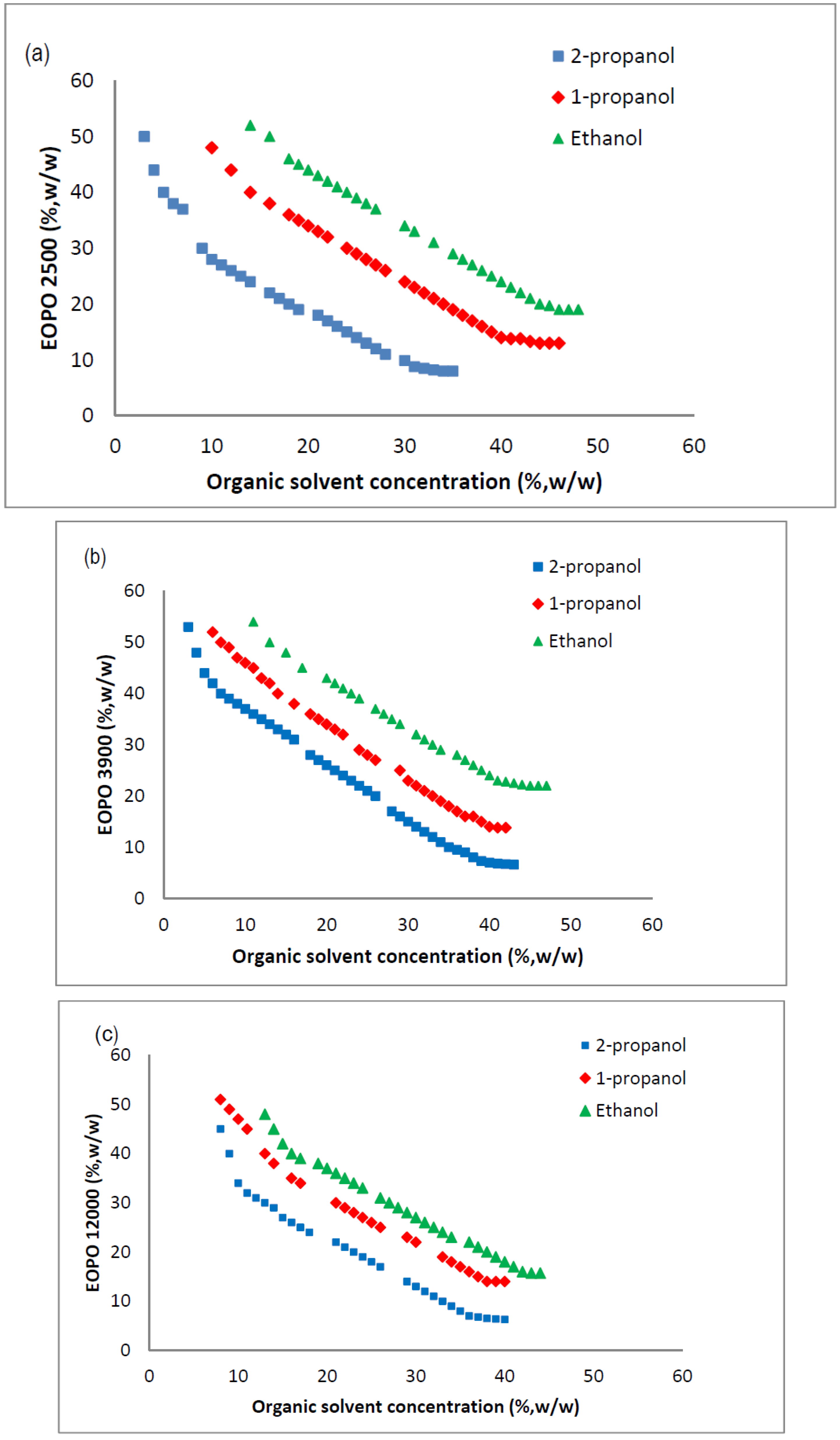
2.4. Influence of Crude Load on Amylase Partition Behavior
| Crude load (%,w/w) | Tie line length | Purification fold | Yield (%) |
|---|---|---|---|
| 15 | 36.4 | 2.31 ± 1.2 a | 74.3 ± 0.5 a |
| 25 | 40.1 | 8.06 ± 0.5 b | 85.6 ± 0.2 b |
| 35 | 48.3 | 4.07 ± 1.1 c | 63.4 ± 1.1 c |
| 45 | 52.3 | 3.02 ± 1.3 c | 55.3 ± 0.3 d |
| 55 | 69.2 | 2.8 ± 2.2 c | 48.2 ± 0.4 d |
2.5. Influence of Volume Ratio on Partitioning of Amylase

2.6. Influence of pH on Amylase Partitioning

2.7. Recycling of Temperature Induced Top Phase Polymer


2.8. Amylase Recovery

3. Experimental
3.1. Chemicals and Plant Material
3.2. Extraction of Amylase
3.3. Preparation of Thermo-Separating ATPS
3.4. Preparation of Phase Diagram (Binodal Curve)
3.5. Recycling of Copolymer

3.6. Amylase Activity Assay
3.7. Protein Concentration Determination
3.8. Acetone Precipitation
3.9. Calculation of Partition Parameters





3.10. Determination of Purity of Amylase Using (SDS-PAGE)
3.11. Statistical Design and Analysis
4. Conclusions
Abbreviations
| ATPS | Aqueous Two Phase System |
| PEG | Polyethylene Glycol |
| EOPO | Ethylene Oxide-Propylene Oxide |
| BSA | Bovine Serum Albumin |
| DNS | 3,5-dinitrosalicylic acid |
| SDS-PAGE | Sodium Dodecyl Sulfate Gel Electrophoresis |
| CRD | Completely Randomized Design |
| ANOVA | Analysis of Variance |
| LSD | Least Significant Difference |
| SD | Standard Deviation |
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Becker, J.S.; Thomas, O.R.T.; Franzre, M. Protein separation with magnetic adsorbents in micellar aqueous two-phase systems. Sep. Purif. Technol. 2009, 65, 46–53. [Google Scholar] [CrossRef]
- Chavez-Santoscoy, A.; Benavides, J.; Vermaas, W.; Rito-Palomares, M. Application of aqueous two-phase systems for the potential extractivefermentation of cyanobacterial products. Chem. Eng. Technol. 2010, 33, 177–182. [Google Scholar] [CrossRef]
- Ashipala, O.K.; He, Q. Optimization of fibrinolytic enzyme production by Bacillus subtilis DC-2 in aqueous two-phase system (poly-ethylene glycol 4000 and sodium sulfate). Bioresour. Technol. 2008, 99, 4112–4119. [Google Scholar] [CrossRef]
- Persson, J.; Kaul, A.; Tjerneld, F. Polymer recycling in aqueous two-phase extractions using thermoseparating ethylene oxide-propylene oxide copolymers. J. Chromatogr. B 2000, 743, 115–126. [Google Scholar] [CrossRef]
- Luechau, F.; Ling, T.C.; Lyddiatt, A. Partition of plasmid DNA in polymer-salt aqueous two-phase systems. Sep. Purif. Technol. 2009, 66, 397–404. [Google Scholar] [CrossRef]
- Li, M.; Kim, J.W.; Peeples, T.L. Amylase partitioning and extractive bioconversion of starch using thermo-separating aqueous two-phase systems. J. Biotechnol. 2002, 93, 15–26. [Google Scholar] [CrossRef]
- Sinha, J.; Dey, P.K.; Panda, T. Aqueous two-phase: The system of choice for extractive fermentation. Appl. Microbiol. Biotechnol. 2000, 54, 476–486. [Google Scholar] [CrossRef]
- Miao, S.; Chen, J.; Cao, X. Preparation of a novel thermo-sensitive copolymer forming recyclable aqueous two-phase systems and its application in bioconversion of Penicillin G. Sep. Purif. Technol. 2010, 75, 156–164. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process. Biochem. 2000, 38, 16–19. [Google Scholar]
- Goyal, N.; Gupta, J.K.; Soni, S.K. A novel raw starch digesting thermostable α-amylase from Bacillis sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb. Technol. 2005, 37, 723–734. [Google Scholar] [CrossRef]
- Hong, C.Y.; Cheng, K.J.; Tseng, T.H.; Wang, C.S.; Liu, L.F.; Yu, S.M. Production of two highly active bacterial phytases with broad pH optima in germinated transgenic rice seeds. Transgenic Res. 2004, 13, 29–39. [Google Scholar] [CrossRef]
- Lim, H.; Tan, C.P.; Karim, R.; Ariffin, A.Z.; Bakar, J. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus. Food Chem. 2010, 119, 1326–1331. [Google Scholar] [CrossRef]
- Dembczynski, R.; Bialas, W.; Regulski, K.; Jankowski, T. Lysozyme extraction from hen egg white in an aqueous two-phase system composed of ethylene oxide–propylene oxide thermoseparating copolymer and potassium phosphate. Process. Biochem. 2010, 45, 369–374. [Google Scholar] [CrossRef]
- Benavides, J.; Rito-Palomares, M.; Asenjo, J.A. Aqueous Two Phase Systems. In Comprehensive Biotechnology, 2nd ed.; Murray, M.Y., Ed.; Academic Press: Burlington, MA, USA, 2011; Volume 2, pp. 697–713. [Google Scholar]
- Bonomo, R.C.F.; Minim, L.A.; Coimbra, J.S.R.; Fontan, R.C.I.; Mendes da Silva, L.H.; Minim, V.P.R. Hydrophobic interaction adsorption of whey proteins: Effect of temperature and salt concentration and thermodynamic analysis. J. Chromatogr. B 2006, 844, 6–11. [Google Scholar] [CrossRef]
- Chien, W.O.; Siew, L.H.; Mazlina, M.K.; Arbakariya, A.; Tau, C.L. Extractive fermentation using aqueous two-phase systems for integrated production and purification of extracellular lipase derived from Burkholderia pseudomallei. Process. Biochem. 2011, 46, 68–73. [Google Scholar]
- Chethana, S.; Nayak, C.A.; Raghavarao, K.S.M.S. Aqueous two phase extraction for purification and concentration of betalains. Food Eng. 2007, 81, 679–687. [Google Scholar] [CrossRef]
- Selvakumar, P.; Ling, T.C.; Walker, S.; Lyddiatt, A. A practical implementation and exploitation of ATPS for intensive processing of biological feedstock: A novel approach for heavily biological feedstock loaded ATPS. Sep. Purif. Technol. 2010, 75, 323–331. [Google Scholar] [CrossRef]
- Show, P.L.; Tan, C.P.; Anuar, M.S.; Ariff, A.; Yusoff, Y.A.; Chen, S.K.; Ling, T.C. Primary recovery of lipase and recycling phase components in aqueous two phase system. Biochem. Eng. 2012, 60, 74–80. [Google Scholar] [CrossRef]
- Saravanan, S.; Rao, J.R.; Nair, B.U.; Ramasami, T. Aqueous two-phase poly(ethylene glycol)–poly(acrylic acid) system for protein partitioning: Influence of molecular weight, pH and temperature. Process. Biochem. 2008, 43, 905–911. [Google Scholar]
- Raja, S.; Murty, V.R.; Thivaharan, V.; Rajasekar, V.; Ramesh, V. Aqueous two phase systems for the recovery of biomolecules-a review. Sci. Technol. 2011, 1, 7–16. [Google Scholar]
- Ramyadevi, D.; Subathira, A.; Saravanan, S. Aqueous two-phase poly (ethylene glycol) – Maltodextrin system for protein partitioning from shrimp waste: Influence of molecular weight and pH. Int. J. Environ. Sci. 2012, 2, 2462–2469. [Google Scholar]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Kammoun, R.; Naili, B.; Bejar, S. Application of a statistical design to the optimization of parameters and culture medium for alpha-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour. Technol. 2008, 99, 5602–5609. [Google Scholar] [CrossRef]
- Jiang, L.; He, L.; Fountoulakis, M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J. Chromatogr. A 2004, 1023, 317–320. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Mirjana, G.A. Partitioning of pectinase produced by Polyporus squamosus in aqueous two phase system polyethylene glycol 4000 crude dextran at different initial pH values. Carbohydr. Polym. 2004, 56, 295–300. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1999, 2, 680–685. [Google Scholar]
- Sample Availability: Samples of pitaya (Hylocereus polyrhizus) peel are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amid, M.; Manap, Y.; Zohdi, N.K. A Novel Aqueous Two Phase System Composed of a Thermo-Separating Polymer and an Organic Solvent for Purification of Thermo-Acidic Amylase Enzyme from Red Pitaya (Hylocereus polyrhizus) Peel. Molecules 2014, 19, 6635-6650. https://doi.org/10.3390/molecules19056635
Amid M, Manap Y, Zohdi NK. A Novel Aqueous Two Phase System Composed of a Thermo-Separating Polymer and an Organic Solvent for Purification of Thermo-Acidic Amylase Enzyme from Red Pitaya (Hylocereus polyrhizus) Peel. Molecules. 2014; 19(5):6635-6650. https://doi.org/10.3390/molecules19056635
Chicago/Turabian StyleAmid, Mehrnoush, Yazid Manap, and Nor Khanani Zohdi. 2014. "A Novel Aqueous Two Phase System Composed of a Thermo-Separating Polymer and an Organic Solvent for Purification of Thermo-Acidic Amylase Enzyme from Red Pitaya (Hylocereus polyrhizus) Peel" Molecules 19, no. 5: 6635-6650. https://doi.org/10.3390/molecules19056635




