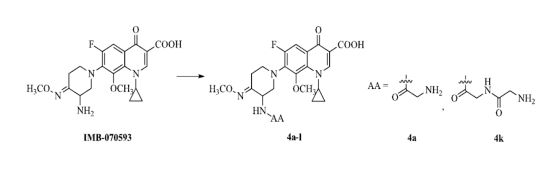
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of 3a–j
To a solution of amino acids
1a–
j (20 mmol) in methanol (90 mL) was added di-
tert-butyl carbonate (8.73 g, 40 mmol) and triethylamine (11.1 mL, 80 mmol). The reaction mixture was heated to refluxing and stirred for 3 h at the same temperature, and concentrated under reduced pressure. The residue was diluted with water (40 mL), adjusted to pH 2.0–3.0 with 2 N HCl at 0–5 °C, and then extracted with ethyl acetate (50 mL × 3). The combined extracts were washed with saturated brine (30 mL), dried over anhydrous Na
2SO
4, and then concentrated under reduced pressure to provide
2a–
j as white solids [
18,
19,
20,
21,
22,
23] (64.8%–91.4%). A mixture of IMB-070593 (2.08 g, 4.97 mmol),
2a–
j (5.71 mmol), dicyclohexylcarbodiimide (1.18 g, 5.71 mmol) and dry dichloromethane (42 mL) was stirred at room temperature for 1 h and filtered. The filtrate was concentrated under reduced pressure, and the residue was treated with diethyl ether (20 mL), and then filtered. The solid was purified by column chromatography (silica gel) eluted with dichloromethane and methanol (v:v = 55:1) to afford the title compounds
3a–
j (36%–79%, from
1a–
j) as white or yellow solids.
7-(3-(2-(tert-Butoxycarbonylamino)acetamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3a). Obtained from 2a and IMB-070593 as a white solid (86.1%), m.p.: 139–140 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.68 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.90 (d, J = 11.7 Hz, 1H, C5-H), 7.12 (d, J = 5.4 Hz, 1H), 5.09 (s, 1H), 4.70–4.59 (m, 1H), 4.18–4.14 (m, 1H), 4.04–4.01 (m, 1H), 3.92 (s, 3H, CH3O-N), 3.85 (d, J = 5.4 Hz, 1H), 3.79 (s, 3H, CH3O-C), 3.72 (q, J = 6.9 Hz, 1H), 3.62 (d, J = 9.0 Hz, 1H), 3.34 (d, J = 14.2 Hz, 1H), 3.26 (t, J = 11.9 Hz, 1H), 3.04 (t, J = 10.3 Hz, 1H), 2.34–2.32 (m, 1H), 1.47 (s, 9H, Boc), 1.30–1.19 (m, 2H, cyclopropyl CH2), 1.12–0.91 (m, 2H, cyclopropyl CH2). MS-ESI (m/z): 576.31(M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)propanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3b). Obtained from 2b as a off-white solid (84.6%), m.p.: 150–152 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.68 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.91 (d, J = 11.6 Hz, 1H, C5-H), 7.27–7.07 (m, 1H), 4.98 (s, 1H), 4.63 (s, 1H), 4.21 (s, 1H), 4.22–4.10 (m, 1H), 4.03 (s, 1H), 3.92 (d, J = 2.6 Hz, 3H, CH3O-N), 3.81 (s, 3H, CH3O-C), 3.61 (d, J = 7.7 Hz, 1H), 3.36–3.24 (m, 2H), 3.03 (t, J = 10.8 Hz, 1H), 2.32 (s, 1H), 1.46 (d, J = 6.8 Hz, 9H, Boc), 1.38–1.26 (m, 3H, CH3), 1.25–1.13 (m, 2H, cyclopropyl CH2), 1.01 (s, 2H, cyclopropyl CH2). MS-ESI (m/z): 590.32(M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)-3-methylbutanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3c). Obtained from 2c as a white solid (78.6%), m.p.: 130–131 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.69 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.91 (d, J = 11.6 Hz, 1H, C5-H), 7.01–6.89 (m, 1H), 5.06 (s, 1H), 4.73–4.58 (m, 1H), 4.19–4.08 (m, 1H), 4.07–4.01 (m, 1H), 3.92 (d, J = 8.3 Hz, 3H, CH3O-N), 3.80 (s, 3H, CH3O-C), 3.61 (s, 1H), 3.48 (q, J = 7.0 Hz, 1H), 3.39–3.20 (m, 2H), 3.06–2.99 (m, 1H), 2.31 (s, 1H), 2.21–2.08 (m, 1H, CH(CH3)2), 1.45 (d, J = 2.3 Hz, 9H, Boc), 1.23–1.21(m, 2H, cyclopropyl CH2), 1.09–0.88 (m, 8H, cyclopropyl CH2, CH(CH3)2). MS-ESI (m/z): 618.65 (M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)-4-methylpentanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3d). Obtained from 2d as a yellow solid (58.2%), m.p.: 142–143 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm) 14.67 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.91 (d, J = 11.6 Hz, 1H, C5-H), 7.26–7.02 (m, 1H), 4.88 (d, J = 19.8 Hz, 1H), 4.64 (s, 1H), 4.14 (d, J = 17.1 Hz, 1H), 4.06–4.00 (m, 1H), 3.92 (d, J = 9.4 Hz, 3H, CH3O-N), 3.80 (d, J = 2.6 Hz, 3H, CH3O-C), 3.61 (s, 1H), 3.46 (d, J = 10.3 Hz, 1H), 3.39–3.21 (m, 2H), 3.03 (t, J = 10.5 Hz, 1H), 2.38–2.24 (m, 1H), 1.94 (d, J = 9.8 Hz, 1H), 1.70 (s, 2H), 1.45 (d, J = 6.4 Hz, 9H, Boc), 1.38–1.30 (m, 1H), 1.27 (d, J = 10.0 Hz, 1H), 1.21–1.13 (m, 2H, cyclopropyl CH2), 1.04–1.00 (m, 1H), 0.98–0.95 (m, 5H). MS-ESI (m/z): 632.63 (M+H)+.
7-(3-((S)-1-(tert-Butoxycarbonyl)pyrrolidine-2-carboxamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3e). The title compound 3e was obtained from 2e as a off-white solid (83.6%), m.p.: 186–187 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.67 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.91 (d, J = 11.5 Hz, 1H, C5-H), 7.00 (s, 1H), 4.63 (s, 1H), 4.22 (s, 1H), 4.10 (d, J = 15.4 Hz, 1H), 4.03 (d, J = 3.9 Hz, 1H), 3.91 (d, J = 2.6 Hz, 3H, CH3O-N), 3.81 (d, J = 4.8 Hz, 3H, CH3O-C), 3.70–3.17 (m, 6H), 3.03 (s, 1H), 2.47–2.10 (m, 2H), 2.00–1.84 (m, 2H), 1.46 (d, J = 10.9 Hz, 9H, Boc), 1.29–1.20 (m, 2H, cyclopropyl CH2), 1.00 (d, J = 4.4 Hz, 2H, cyclopropyl CH2). MS-ESI (m/z): 616.67 (M+H)+.
7-(3-((2S,3S)-2-(tert-Butoxycarbonylamino)-3-methylpentanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3f). Obtained from 2f as a white solid (96.5%), m.p. : 126–127 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.68 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.91 (d, J = 11.6 Hz, 1H, C5-H), 6.99 (d, J = 5.9 Hz, 1H), 5.08 (s, 1H), 4.67 (d, J = 4.1 Hz, 1H), 4.21–3.98 (m, 3H), 3.92 (d, J = 8.5 Hz, 3H, CH3O-N), 3.80 (s, 3H, CH3O-C), 3.61 (s, 1H), 3.35 (d, J = 14.1 Hz, 1H), 3.26 (s, 1H), 3.03 (d, J = 9.6 Hz, 1H), 2.30 (s, 1H), 1.90–1.82 (m, 2H), 1.46 (s, 9H, Boc), 1.29–1.16 (m, 3H), 1.01 (s, 2H), 0.95–0.91 (m, 6H). MS-ESI (m/z): 632.35 (M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)-3-phenylpropanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3g). Obtained from 2g as a white solid (79.1%), m.p.: 149–150 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.68 (s, 1H, COOH), 8.84 (s, 1H, C2-H), 7.92 (d, J = 11.6 Hz, 1H, C5-H), 7.40–7.22 (m, 5H, ph-H), 6.90–6.77 (m, 1H), 5.07–5.01 (m, 1H), 4.56 (s, 1H), 4.40 (s, 1H), 4.16–3.97 (m, 2H), 3.85 (d, J = 10.0 Hz, 3H, CH3O-N), 3.79 (s, 3H, CH3O-C), 3.56 (s, 1H), 3.31–2.75 (m, 5H), 2.28 (t, J = 12.4 Hz, 1H), 1.43 (s, 9H, Boc), 1.28–1.20 (m, 2H, cyclopropyl CH2), 1.03 (d, J = 11.3 Hz, 2H, cyclopropyl CH2). MS-ESI (m/z): 666.31 (M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)-3-hydroxypropanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3h). Obtained from 2h as a off-white solid (83.3%), m.p. : 133–135 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.64 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.92 (d, J = 11.6 Hz, 1H, C5-H), 7.15 (s, 1H), 5.48 (s, 1H), 4.84–4.61 (m, 1H), 4.23 (s, 1H), 4.17–3.97 (m, 3H), 3.93 (d, J = 11.7 Hz, 3H, CH3O-N), 3.80 (d, J = 2.6 Hz, 3H, CH3O-C), 3.70–3.68 (m, 1H), 3.64–3.43 (m, 1H), 3.35–3.25 (m, 2H), 3.12 (t, J = 10.3 Hz, 1H), 2.37–2.31 (m, 1H), 1.91 (brs, 1H, OH), 1.47 (d, J = 10.2 Hz, 9H, Boc), 1.29–1.19 (m, 2H, cyclopropyl CH2), 1.03 (t, J = 7.7 Hz, 2H, cyclopropyl CH2). MS-ESI (m/z): 606.24 (M+H)+.
7-(3-((S)-2-(tert-Butoxycarbonylamino)-3-(4-hydroxyphenyl)propanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3i). Obtained from 2i as a off-white solid (43.9%), m.p.: 151–153 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.73 (s, 1H, COOH), 8.82 (s, 1H, C2-H), 7.89–7.84 (m, 1H, C5-H), 7.02 (d, J = 8.1 Hz, 2H), 6.98–6.76 (m, 1H), 6.72 (d, J = 8.3 Hz, 2H), 5.14 (d, J = 35.9 Hz, 1H), 4.56 (s, 1H), 4.37 (s, 1H), 4.06 (d, J = 12.0 Hz, 2H), 3.87 (d, J = 10.9 Hz, 3H, CH3O-N), 3.80 (d, J = 4.7 Hz, 3H, CH3O-C), 3.76–3.46 (m, 2H), 3.22 (d, J = 11.9 Hz, 2H), 3.03–2.89 (m, 2H), 2.38–2.23 (m, 1H), 1.44 (s, 9H, Boc), 1.26–1.19 (m, 2H, cyclopropyl CH2), 1.12–0.94 (m, 2H, cyclopropyl CH2). MS-ESI (m/z): 682.22 (M+H)+.
7-(3-((2S,3R)-2-(tert-Butoxycarbonylamino)-3-hydroxybutanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3j). Obtained from 2j as a white solid (47.7%), m.p.: 134–136 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.64 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.92 (d, J = 11.6 Hz, 1H, C5-H), 7.33 (s, 1H), 5.42 (s, 1H), 4.74–4.65 (m, 1H), 4.42–4.33 (m, 1H), 4.17–4.03 (m, 3H), 3.92 (t, J = 4.3 Hz, 3H, CH3O-N), 3.80 (s, 3H, CH3O-C), 3.61 (s, 1H), 3.29 (dd, J = 22.8, 13.8 Hz, 2H), 3.11 (t, J = 11.4 Hz, 1H), 2.43–2.26 (m, 1H), 1.80 (brs, 1H, OH), 1.46 (d, J = 11.6 Hz, 9H, Boc), 1.26–1.20 (m, 5H, cyclopropyl CH2, CH3CH), 1.01 (s, 2H, cyclopropyl CH2). MS-ESI (m/z): 620.21 (M+H)+.
3.2.2. General Procedure for the Synthesis of 4a–j
To a stirring solution of trifluoroacetic acid (8.0 mL) was added 3a–j (1.80 mmol) in portions over a period of 0.5 h at −5–0 °C and stirred for 3 h at the same temperature, and then concentrated under reduced pressure. The residue was treated with diethyl ether (10 mL) and then filtered. The solid was dissolved in methanol (2 mL) and adjusted to pH 7.0 with the ammonia water, and then extracted with dichloromethane (30 mL × 3). The combined extracts were washed with saturated brine (10 mL) and dried over anhydrous Na2SO4, and then concentrated under reduced pressure to provide the title compounds 4a–j.
7-(3-(2-Aminoacetamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4a). Obtained from 3a as a yellow solid (71.8%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.83 (s, 1H, C2-H), 8.14 (s, 1H), 7.90 (d, J = 8.9 Hz, 1H, C5-H), 4.71 (s, 1H), 4.03–3.81 (m, 6H), 3.62 (s, 1H), 3.49–3.12 (m, 6H), 2.41 (s, 2H), 1.25–1.21 (m, 2H, cyclopropyl CH2), 1.02 (d, J = 17.5 Hz, 2H, cyclopropyl CH2). 13C-NMR (150 MHz, DMSO-d6) δ 176.33, 172.48, 165.59, 156.28, 154.37, 150.61, 146.23, 138.59(d, J = 12.2 Hz), 134.06, 121.49, 106.69, 106.46, 63.24, 61.33, 55.74, 49.15, 44.50, 40.73, 24.85, 18.55, 9.03, 8.86. MS-ESI (m/z): 476.23(M+H)+. HRMS-ESI (m/z): C22H27O6N5F, Calcd: 476.193899(M+H)+; Found: 476.19389(M+H)+.
7-(3-((S)-2-Aminopropanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4b). Obtained from 3b as a yellow solid (88.3%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.81 (s, 1H, C2-H), 8.38–8.00 (m, 1H), 7.87 (d, J = 11.7 Hz, 1H, C5-H), 4.67 (s, 1H), 4.06 (s, 2H), 3.93 (s, 3H, CH3O-N), 3.82 (s, 3H, CH3O-C), 3.57 (d, J = 13.5 Hz, 2H), 3.31 (d, J = 11.1 Hz, 2H), 3.11 (s, 1H), 2.55–2.30 (m, 1H), 1.38–1.33 (m, 2H), 1.26–1.19 (m, 3H), 1.03 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 177.15, 175.65, 166.78, 157.70, 153.47, 150.12, 146.20, 139.02 (d, J = 12 Hz), 133.91, 122.77, 108.40, 107.96, 62.84, 62.06, 56.65, 51.00, 50.64, 50.24, 40.70, 25.67, 21.77, 9.86, 9.53. MS-ESI (m/z): 490.25 (M+H)+.HRMS-ESI (m/z): C23H29O6N5F, Calcd: 490.20964 (M+H)+; Found: 490.20968 (M+H)+.
7-(3-((S)-2-Amino-3-methylbutanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4c). Obtained from 3c as a light yellow solid (70.0%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 8.70 (s, 1H, C2-H), 8.29–8.24 (m, 1H), 7.77 (d, J = 10.6 Hz, 1H, C5-H), 4.59–4.55 (m, 1H), 4.16 (s, 1H), 3.83 (s, 3H, CH3O-N), 3.76 (d, J = 5.2 Hz, 3H, CH3O-N), 3.73–3.58 (m, 1H), 3.56–3.41 (m, 1H), 3.28–3.16 (m, 1H), 3.03 (d, J = 3.5 Hz, 2H), 2.60–2.50 (m, 1H), 1.98–1.94 (m, 1H), 1.91–1.72 (m, 1H), 1.21–0.91 (m, 4H, 2 × cyclopropyl CH2), 0.87 (d, J = 6.8 Hz, 1H), 0.80 (d, J = 6.8 Hz, 2H), 0.74–0.70 (m, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 176.30, 174.16, 165.61, 156.74, 154.45, 150.62, 146.08(d, J = 18 Hz), 138.60, 134.04, 121.33, 106.65, 106.42, 63.11, 61.27, 59.66, 55.47, 49.25, 40.71, 30.30, 24.76, 19.29, 16.65, 16.53, 8.98, 8.88. MS-ESI (m/z): 518.35 (M+H)+.HRMS-ESI (m/z): C25H33O6N5F, Calcd: 518.24094 (M+H)+; Found: 518.24080 (M+H)+.
7-(3-((S)-2-Amino-4-methylpentanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4d). Obtained from 3d as a yellow solid (71.5%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 8.71 (s, 1H, C2-H), 8.46–8.23 (m, 1H), 7.77 (d, J = 10.6 Hz, 1H, C5-H), 4.55–4.49 (m, 1H), 4.17 (s, 1H), 3.83 (s, 3H, CH3O-N), 3.76 (d, J = 8.3 Hz, 3H, CH3O-C), 3.69 (s, 1H), 3.48–3.21 (m, 3H), 3.03 (t, J = 16.1 Hz, 1H), 2.64 (d, J = 6.4 Hz, 1H), 1.72 (s, 1H), 1.56 (s, 1H), 1.41 (s, 1H), 1.35–0.96 (m, 5H), 0.86–0.81 (m, 3H), 0.77–0.73 (m, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 176.31, 175.04, 165.60, 156.72, 154.44, 150.60, 146.02 (d, J = 33 Hz), 138.66, 134.03, 121.43, 106.65, 106.42, 63.13, 61.27, 55.51, 52.99, 49.16, 44.07, 40.70, 24.87, 23.97, 23.12, 23.03, 21.85, 8.97, 8.93. MS-ESI (m/z): 532.40 (M+H)+. HRMS-ESI (m/z): C26H35O6N5F, Calcd: 532.25659 (M+H)+; Found: 532.25671 (M+H)+.
7-(4-(methoxyimino)-3-((S)-pyrrolidine-2-carboxamido)piperidin-1-yl)-1-Cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4e). Obtained from 3e as a yellow solid (66.8%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.81 (s, 1H, C2-H), 8.45–8.39 (m, 1H), 7.87 (d, J = 11.7 Hz, 1H, C5-H), 4.67 (s, 1H), 4.04–3.98 (m, 2H), 3.92 (d, J = 7.8 Hz, 3H, CH3O-N), 3.81 (s, 4H), 3.57 (d, J = 12.5 Hz, 1H), 3.31–3.27 (m, 2H), 3.15 (t, J = 10.7 Hz, 1H), 3.05–2.93 (m, 2H), 2.51–2.31 (m, 1H), 2.15–2.10 (m, 1H), 2.00–1.95 (m, 1H), 1.88–1.56 (m, 2H), 1.24–1.19 (m, 2H, cyclopropyl CH2), 1.02 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 177.12, 175.17, 166.76, 157.61, 153.61, 150.11, 146.10, 138.97 (d, J = 12 Hz), 133.91, 122.64, 108.41, 107.97, 62.81, 61.96, 60.82, 56.59, 50.52, 49.88, 47.37, 40.68, 30.92, 26.21, 25.61, 9.86, 9.53. MS-ESI (m/z): 516.36 (M+H)+.HRMS-ESI (m/z): C25H31O6N5F, Calcd: 516.22529 (M+H)+; Found: 516.22545 (M+H)+.
7-(3-((2S,3S)-2-Amino-3-methylpentanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4f). Obtained from 3f as a yellow solid (88.3%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.73 (d, J = 3.5 Hz, 1H, C2-H), 7.91–7.57 (m, 1H, C5-H), 7.46 (s, 1H), 4.71 (s, 1H), 4.13–4.04 (m, 3H), 3.90 (d, J = 6.9 Hz, 3H, CH3O-N), 3.79 (s, 3H, CH3O-C), 3.57–3.47 (m, 1H), 3.34–3.19 (m, 2H), 2.41 (s, 1H), 2.13 (s, 1H), 1.63–1.58 (m, 1H), 1.22 (d, J = 6.4 Hz, 4H), 1.06–0.97 (m, 6H), 0.92 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 176.74, 175.34, 166.81, 154.83, 153.05, 150.25, 145.93(d, J = 12 Hz), 138.91, 134.05, 122.08, 107.94, 107.61, 63.12, 62.15, 58.19, 55.96, 50.94, 50.48, 40.87, 37.01, 25.38, 14.86, 11.75, 9.76. MS-ESI (m/z): 532.33 (M+H)+.HRMS-ESI (m/z): C26H35O6N5F, Calcd: 532.25659 (M+H)+; Found: 532.25653 (M+H)+.
7-(3-((S)-2-Amino-3-phenylpropanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4g). Obtained from 3g as a yellow solid (82.4%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 8.72 (s, 1H, C2-H), 8.28 (d, J = 4.6 Hz, 1H), 7.90–7.70 (m, 1H, C5-H), 7.40–6.90 (m, 5H, ph-H), 4.54 (s, 1H), 4.17 (s, 1H), 3.83 (d, J = 8.0 Hz, 3H, CH3O-N), 3.76–3.66 (m, 4H), 3.47 (d, J = 5.7 Hz, 2H), 3.31 (s, 2H), 3.23–3.07 (m, 1H), 2.96–2.91 (m, 2H), 2.74–2.51 (m, 1H), 1.26–0.83 (m, 4H, 2 × cyclopropyl CH2). 13C-NMR (100 MHz, DMSO-d6) δ 176.29, 173.76, 165.66, 156.71, 154.33, 150.64, 146.24, 138.35(d, J = 29 Hz), 134.00, 129.24, 128.02, 126.01, 121.57, 106.69, 106.46, 63.14, 61.30, 56.00, 55.68, 49.25, 40.68, 40.45, 24.70, 16.37, 8.95. MS-ESI (m/z): 566.40 (M+H)+. HRMS-ESI (m/z): C29H33O6N5F, Calcd: 566.24094 (M+H)+; Found: 566.24108 (M+H)+.
7-(3-((S)-2-Amino-3-hydroxypropanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4h). Obtained from 3h as a yellow solid (66.2%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 8.71 (s, 1H, C2-H), 8.36 (d, J = 5.1 Hz, 1H), 7.78 (d, J = 8.0 Hz, 1H, C5-H), 4.76 (s, 1H), 4.56–4.52 (m, 1H), 4.17 (s, 1H), 3.84 (d, J = 0.9 Hz, 3H, CH3O-N), 3.81–3.70 (m, 4H), 3.60–3.35 (m, 4H), 3.26–3.25 (m, 2H), 3.11–2.96 (m, 1H), 2.56–2.51 (m, 1H), 1.13–0.96 (m, 4H, 2 × cyclopropyl CH2). 13C-NMR (100 MHz, DMSO-d6) δ 176.36, 172.77, 165.60, 156.82, 154.37, 150.61, 146.29 (d, J = 11 Hz), 138.67, 134.05, 121.49, 106.69, 106.42, 64.90, 63.18, 61.34, 56.76, 55.65, 49.40, 40.75, 24.85, 15.16, 8.92. MS-ESI (m/z): 506.28 (M+H)+.HRMS-ESI (m/z): C23H29O7N5F, Calcd: 506.20455 (M+H)+; Found: 506.20465 (M+H)+.
7-(3-((S)-2-Amino-3-(4-hydroxyphenyl)propanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4i). Obtained from 3i as a light yellow solid (83.4%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 9.11 (d, J = 15.9 Hz, 1H), 8.71 (d, J = 4.5 Hz, 1H, C2-H), 8.23 (t, J = 8.0 Hz, 1H), 7.79 (dd, J = 11.8, 6.1 Hz, 1H, C5-H), 6.95 (t, J = 7.6 Hz, 2H), 6.59 (dd, J = 14.6, 8.3 Hz, 2H), 4.53 (s, 1H), 4.18 (dd, J = 6.8, 3.8 Hz, 1H), 3.83 (d, J = 7.8 Hz, 3H, CH3O-N), 3.74 (d, J = 10.8 Hz, 3H, CH3O-C), 3.67 (d, J = 7.8 Hz, 1H), 3.54–3.44 (m, 1H), 3.42–3.34 (m, 2H), 3.26–3.16 (m, 1H), 3.15–2.89 (m, 2H), 2.79 (d, J = 11.0 Hz, 1H), 2.65–2.53 (m, 1H), 1.16–1.01 (m, 4H, 2 × cyclopropyl CH2). 13C-NMR (100 MHz, DMSO-d6) δ 176.40, 173.94, 165.64, 156.74, 155.78, 154.30, 150.63, 146.26, 138.72(d, J = 38 Hz), 134.07, 130.27, 130.09, 128.33, 127.95, 121.53, 114.88, 106.69, 106.48, 63.15, 61.31, 56.18, 55.80, 49.71, 49.24, 40.75, 24.87, 15.15, 9.06, 8.96. MS-ESI (m/z): 582.30 (M+H)+.HRMS-ESI (m/z): C29H33O7N5F, Calcd: 582.23585 (M+H)+; Found: 582.23593 (M+H)+.
7-(3-((2S,3R)-2-Amino-3-hydroxybutanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4J). Obtained from 3J as a yellow solid (73.3%). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) 8.71 (s, 1H, C2-H), 8.38 (d, J = 7.0 Hz, 1H), 7.78 (dd, J = 11.8, 3.1 Hz, 1H, C5-H), 4.63–4.50 (m, 1H), 4.22–4.10 (m, 1H), 3.91–3.80 (m, 4H), 3.79–3.59 (m, 4H), 3.51–3.47 (m, 1H), 3.31–3.12 (m, 2H), 3.10–3.02 (m, 1H), 2.99 (d, J = 4.1 Hz, 1H), 2.60–2.51 (m, 1H), 1.29–1.23 (m, 1H), 1.13–1.11 (m, 2H), 1.04–0.97 (m, 3H), 0.91–0.73 (m, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 176.34, 173.04, 165.60, 156.84, 154.39, 150.61, 146.10, 138.56 (d, J = 38 Hz), 134.06, 122.24, 106.66, 106.42, 67.14, 63.17, 61.32, 60.19, 55.60, 49.47, 39.94, 24.85, 20.08, 19.94, 8.94. MS-ESI (m/z): 520.28 (M+H)+. HRMS-ESI (m/z): C24H31O7N5F, Calcd: 520.22020 (M+H)+; Found: 520.22083 (M+H)+.
3.2.3. General Procedure for the Synthesis of 3k–l
A mixture of 4a–b (0.82 mmol), 2a–b (0.82 mmol), dicyclohexylcarbodiimide (0.17 g, 0.82 mmol) and dry dichloromethane (20 mL) was stirred at room temperature for 4 h, filtered and concentrated under reduced pressure. The solid was treated with diethyl ether (3 mL) and filtered. The solid was purified by column chromatography (silica gel) eluted with dichloromethane and methanol (v:v = 60:1) to afford the title compounds 3k–l.
7-(3-(2-(2-(tert-Butoxycarbonylamino)acetamido)acetamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3k). Obtained from 4a and 2a as a light yellow solid (30.8%), m.p.: 122–124 °C.1H-NMR (400 MHz, CDCl3) δ (ppm) 14.71 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.90 (d, J = 11.6 Hz, 1H, C5-H), 6.97 (d, J = 5.6 Hz, 1H), 6.75 (s, 1H), 5.07 (s, 1H), 4.72–4.56 (m, 1H), 4.11 (d, J = 7.0 Hz, 1H), 4.06–3.95 (m, 3H), 3.93 (s, 3H, CH3O-N), 3.86 (d, J = 3.7 Hz, 2H), 3.78 (s, 3H, CH3O-C), 3.61 (d, J = 10.0 Hz, 1H), 3.34–3.24 (m, 2H), 3.07 (t, J = 10.5 Hz, 1H), 2.42–2.26 (m, 1H), 1.45 (s, 9H, Boc), 1.26–1.21 (m, 2H, cyclopropyl CH2), 1.07–0.98 (m, 2H, cyclopropyl CH2). MS-ESI (m/z): 633.17 (M+H)+.
7-(3-((S)-2-((S)-2-(tert-Butoxycarbonylamino)propanamido)propanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3l). Obtained from 4b and 2b as a yellow solid (71.5%), m.p.: 126–128 °C. 1H-NMR (400 MHz, CDCl3) δ (ppm) 14.68 (s, 1H, COOH), 8.83 (s, 1H, C2-H), 7.90 (d, J = 11.6 Hz, 1H, C5-H), 7.18–6.92 (m, 1H), 6.71 (d, J = 20.8 Hz, 1H), 4.93 (s, 1H), 4.70–4.58 (m, 1H), 4.53–4.48 (m, 1H), 4.16 (s, 1H), 4.16–4.03 (m, 2H), 3.92 (d, J = 4.0 Hz, 3H, CH3O-N), 3.79 (d, J = 1.5 Hz, 3H, CH3O-C), 3.59 (s, 1H), 3.40–3.18 (m, 2H), 3.14–2.97 (m, 1H), 2.34–2.32 (m, 1H), 1.63–1.29 (m, 15H, Boc, 2 × CH3), 1.28–1.16 (m, 2H, cyclopropyl CH2), 1.09–0.87 (m, 2H, cyclopropyl CH2). MS-ESI (m/z): 661.33 (M+H)+.
3.2.4. General Procedure for the Synthesis of 4k–l
To a stirring solution of trifluoroacetic acid (3.0 mL) was added 3k–l (0.47 mmol) in portions over a period of 0.5 h at −5–0 °C, stirred for 2 h at the same temperature and then concentrated under reduced pressure. The residue was treated with diethyl ether (7 mL) and filtered. The solid was dissolved in methanol (1 mL) and adjusted to pH 7.0 with the ammonia water, and then extracted with dichloromethane (50 mL × 3). The combined extracts were washed with saturated brine (10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure to provide the title compounds 4k–l.
7-(3-(2-(2-Aminoacetamido)acetamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4k). Obtained from 3k as a yellow solid (73.3%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.81 (s, 1H, C2-H), 7.98–7.80 (m, 2H), 7.11 (d, J = 5.9 Hz, 1H), 4.75–4.56 (m, 1H), 4.14–4.10 (m, 1H), 4.07–4.00 (m, 2H), 3.97 (d, J = 5.6 Hz, 1H), 3.92 (s, 3H, CH3O-N), 3.79 (s, 3H, CH3O-C), 3.60 (d, J = 8.0 Hz, 1H), 3.52–3.44 (m, 1H), 3.44 (s, 1H), 3.35–3.19 (m, 2H), 3.09 (t, J = 10.7 Hz, 1H), 2.46–2.22 (m, 1H), 1.27–1.18 (m, 2H, cyclopropyl CH2), 1.13–0.95 (m, 2H, cyclopropyl CH2). 13C-NMR (100 MHz, CDCl3) δ 177.07, 173.52, 168.75, 166.74, 157.52, 152.99, 150.12, 146.05, 138.85(d, J = 12 Hz), 133.94, 122.74, 108.33, 107.96, 62.95, 62.11, 56.43, 50.57, 50.15, 44.79, 42.87, 40.70, 25.66, 9.86, 9.53. MS-ESI (m/z): 533.32 (M+H)+.HRMS-ESI (m/z): C24H30O7N6F, Calcd: 533.21545 (M+H)+; Found: 533.21660 (M+H)+.
7-(3-((S)-2-((S)-2-Aminopropanamido)propanamido)-4-(methoxyimino)piperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4l). Obtained from 3l as a yellow solid (34.7%). 1H-NMR (400 MHz, CDCl3) δ (ppm) 8.81 (s, 1H, C2-H), 7.86 (dd, J = 11.6, 6.2 Hz, 1H, C5-H), 7.75 (t, J = 7.8 Hz, 1H), 7.28–7.10 (m, 1H), 4.66–4.62 (m, 1H), 4.56–4.47 (m, 1H), 4.13–4.00 (m, 2H), 3.92 (d, J = 4.6 Hz, 3H, CH3O-N), 3.80 (s, 3H, CH3O-C), 3.68–3.44 (m, 2H), 3.32–3.25 (m, 2H), 3.08 (t, J = 10.4 Hz, 1H), 2.34 (t, J = 12.7 Hz, 1H), 1.44–1.33 (m, 5H), 1.26–1.21 (m, 3H), 1.03 (s, 2H). 13C-NMR (100 MHz, CDCl3) δ 177.11, 175.83, 172.12, 166.76, 159.46, 153.12, 150.11, 146.14(d, J = 12 Hz), 138.80, 133.90, 122.82, 108.39, 107.98, 62.90, 62.07, 56.37, 50.78, 50.60, 48.68, 48.47, 40.69, 25.69, 21.66, 18.13, 9.80, 9.58. MS-ESI (m/z): 561.44 (M+H)+.HRMS-ESI (m/z): C26H34O7N6F, Calcd: 561.24675 (M+H)+; Found: 561.24691 (M+H)+.


 (c, CH3OH)
(c, CH3OH)