Processing of Kansui Roots Stir-Baked with Vinegar Reduces Kansui-Induced Hepatocyte Cytotoxicity by Decreasing the Contents of Toxic Terpenoids and Regulating the Cell Apoptosis Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. UPLC-QTOF/MS Analysis and Identification of the Most Differentiating Components between Kansui (GS-1) and Kansui Stir-Baked with Vinegar (GS-2)

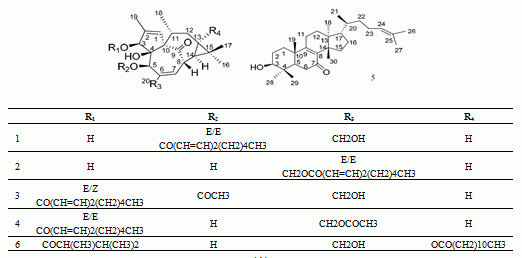
| Peak No. | RT (min) | HR-mass (m/z) | Tolerance (ppm) | Molecular Formula | Assigned Identity | A | Rate of Change (%) | |
|---|---|---|---|---|---|---|---|---|
| GS-1 | GS-2 | |||||||
| 1 | 9.11 | 497.2883 (b) | −4.0 | C30H42O6 | 5-O-(2'E,4'E-decadienoyl)ingenol | 300 | 236 | 24.67 |
| 2 | 9.53 | 497.2889 (e) | −2.8 | C30H42O6 | 20-O-(2'E,4'E-decadienoyl)ingenol | 245 | 195 | 20.41 |
| 3 | 10.91 | 539.3026 (d) | 3.2 | C32H44O7 | 3-O-(2'E,4'Z-decadienoyl)-5-O-acetylingenol | 414 | 355 | 14.25 |
| 4 | 11.29 | 539.3015 (c) | 1.1 | C32H44O7 | 3-O-(2'E,4'E-decadienoyl)-20-O-acetylingenol | 330 | 278 | 15.75 |
| 5 | 12.71 | 439.3562 (f) | −3.2 | C30H48O2 | epi-kansenone | 802 | 683 | 14.84 |
| 6 | 15.69 | 643.4228 (a) | 2.8 | C38H60O8 | 3-O-(2,3-dimethylbutanoyl)-13-O-dodecanoylingenol | 942 | 358 | 62.00 |
2.2. Effects on the Rate of Cell Apoptosis

2.3. Effects on Nuclear Fragmentation and the Accumulation of Mitochondrial Mass

2.4. Effects on Cytochrome c Release from Mitochondria into the Cytoplasm

2.5. Effects on Caspase-9 and Caspase-3 Activation

3. Experimental
3.1. Plant Material
3.2. Preparation of Sample Solutions
3.3. UPLC-QTOF/MS Experiments
3.4. Multivariate Data Analysis for the UPLC-QTOF/MS Data
3.5. Cell Line and Cell Culture
3.6. Flow Cytometry Experiments
3.7. High Content Screening (HCS) Experiments
3.8. Western Blotting Experiments
3.9. Elisa Experiments
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, T.S.; Lin, Y.M.; Haruna, M.; Pan, D.J.; Shingu, T.; Chen, Y.P.; Hsu, H.Y. Antitumor agents, 119. Kansuiohorins A and B, two novel antileukemic diterpene esters from Euphorbia kansui. J. Nat. Prod. 1991; 54, 823–829. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, N.L.; Yao, X.S.; Miyata, S.; Kitanaka, S. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 2002, 65, 1246–1251. [Google Scholar] [CrossRef]
- Wang, L.Y. Study on Chemical Constituents and Biological Activity of Kansui. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2003. [Google Scholar]
- Yu, F.R.; Lian, X.Z.; Guo, H.Y.; McGuire, P.M.; Li, R.D.; Wang, R.; Yu, F.H. Isolation and characterization of methy esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharmacol. 2005, 8, 523–528. [Google Scholar]
- Miyata, S.; Wang, L.Y.; Yoshida, C.; Kitanaka, S. Inhibition of cellular proliferation by diterpenes, topoisomerase II inhibitor. Bioorg. Med. Chem. 2006, 14, 2048–2051. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Y.D.; Tang, H.H.; Wu, F.Y. Study of auxiliary therapeuticc effect of kansui root on patients with severe acute pancreatitis. Chin. J. Mod. Med. 2002, 12, 7–9. [Google Scholar]
- Lu, X.S.; Zhang, Y.; Ai, Y.H.; Li, Y.X. The clinical study of kansui root therapy for severe acute pancreatitis. J. Chin. Phys. 2004, 6, 1444–1447. [Google Scholar]
- OuYang, J.B.; Deng, M.Y.; OuYang, Y.M. Theatpeutic effect on adjuvant treatment of severe acute pancreatitis with Euphorbia kansui Lious. Chin. J. Mod. Med. 2004, 14, 96–97. [Google Scholar]
- Liu, J.H.; Luo, J.; Lu, H.G.; Wu, W.L.; Xue, D.P.; Ma, F.X.; Liu, Y.; Zhang, N. Treatment of postoperative intestinal obstruction on 504 cases with kansui. Chin. J. Crit. Care Med. 1998, 18, 45–46. [Google Scholar]
- Fan, Y.; Cai, D.F.; Gu, X.X.; Wang, G.Y.; Ma, J. Treatment of intestinal obstruction with a large dose of kansui. J. Emerg. Tradit. Chin. Med. 2005, 14, 278–279. [Google Scholar]
- Matsumoto, T.; Cyong, J.C.; Yamada, H. Stimulatory effects of ingenols from Euphorbia kansui on the expression of macrophage Fc receptor. Planta Med. 1992, 58, 255–258. [Google Scholar] [CrossRef]
- Zheng, W.F.; Cui, Z.; Zhu, Q. Cytotoxicity and antiviral activity of the compounds from Euphorbia kansui. Planta Med. 1998, 64, 754–756. [Google Scholar] [CrossRef]
- Yan, X.J.; Li, L.; Li, Z.J.; Li, Y.; Gao, L.; Cao, Y.D.; Tang, Y.P.; Zhang, L. The comparision of dose-effect relationships of crude and vinegar processed Euphorbia kansui with splenic lymphocyte activity and peritoneal macrophage NO release. Chin. Pharmacol. Bull. 2011, 5, 629–632. [Google Scholar]
- Jiang, W.; Wang, X.M.; Tang, Y.P.; Shang, E.X.; Peng, Y.R. Perliminary observation on acute toxicity of different Kansui extraction zebrafish. J. Nanjing Univ. Tradit. Chin. Med. 2012, 1, 53–56. [Google Scholar]
- Yan, X.J.; Zhang, L.; Li, L.; Cao, Y.D.; Li, Z.J.; Tang, Y.P.; Ding, A.W. Study on detoxication of Kansui Radix on normal liver cells LO2 after stir-baking with vinegar. China J. Mater. Med. 2012, 11, 1667–1671. [Google Scholar]
- Huang, W.Q.; Luo, Y. Impact of combining liquorice with kansui root, spurge, seaweed or lilac Daphne flower bud on the function of heart, liver and kidney in rat. Chin. J. Clin. Rehabil. 2004, 8, 3682–3683. [Google Scholar]
- Xiang, L.H.; Chen, Y.P.; Zhang, Z.; Yu, Z.M.; Lv, A.P. Study on the effects of rat organ index in long-term toxicity of 24 kinds of poisonous Chinese Medicine. Chin. J. Basic Tradit. Chin. Med. 2006, 12, 35–36. [Google Scholar]
- Yang, Z.J.; Deng, Y.; Wang, X.; Gao, H.Q. Study on the impact of combining liqurice with kansui root on the on the liver of MDA, GSH-Px in rat. Tradit. Chin. Med. Res. 2006, 19, 15–16. [Google Scholar]
- Zhang, L.; Gao, L.; Yan, X.J.; Cao, Y.D.; Ding, A.W. Effect of Kansui Radix prepared by different processes on LO2 cell cycle and apoptosis. China J. Mater. Med. 2013, 38, 825–830. [Google Scholar]
- Zhang, L.; Gao, L.; Li, Z.J.; Yan, X.J.; Yang, Y.J.; Tang, Y.P.; Cao, Y.D.; Ding, A.W. Bio-Guided Isolation of the Cytotoxic Terpenoids from the Roots of Euphorbia kansui against Human Normal Cell Lines L-O2 and GES-1. Int. J. Mol. Sci. 2012, 13, 11247–11259. [Google Scholar] [CrossRef]
- Geng, T.; Huang, H.Y.; Ding, A.W.; Zhang, L. Irritation and diarrhea effect of different polar parts of Euphorbia kansui and vinegar-preparing Euphorbia kansui. Cent. South Pharm. 2008, 6, 385–388. [Google Scholar]
- Ye, X.Z.; Zhu, D.H.; Shen, D.W. Ultrastructures of LO2 normal adult hepatocytes over successive in vitro cultures. Acta Biol. Exp. Sin. 1980, 13, 362–365. [Google Scholar]
- Ma, M.H.; Biempica, L. The normal human liver cell cytochemical and ultrastructural studies. Am. J. Pathol. 1971, 62, 353–390. [Google Scholar]
- Wang, X.X.; Chen, Y.; Wang, J.J.; Liu, Z.X.; Zhao, S.G. Antitumor activity of a sulfated polysaccharide from Enteromorpha intestinalis targeted against hepatoma through mitochondrial pathway. Tumor Biol. 2013, 13, 1226–1229. [Google Scholar]
- Cui, H.Q.; Wu, F.; Sun, Y.L.; Fan, G.C.; Wang, Q.M. Up-regulation and subcellular localization of hnRNP A2/B1 in the development of hepatocellular carcinoma. BMC Cancer 2010, 10, 356. [Google Scholar] [CrossRef]
- Liu, Z.J.; Ling, K.; Wu, X.; Cao, J.; Liu, B.; Li, S.Y.; Si, Q.; Cai, Y.; Yan, C.; Zhang, Y.; et al. Reduced expression of cenp-e in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 156. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zheng, C.; Xu, W.; Si, Y.X.; Dou, S.F.; Yang, Y.M. Free radical scavenging and hepatoprotective effects of salidroside analogs on CCl4-induced cytotoxicity in LO2 cells. Med. Chem. Res. 2013, 22, 2524–2530. [Google Scholar]
- Jiang, Z.Q.; Yan, X.J.; Bi, L.; Chen, J.P.; Zhou, Q.; Chen, W.P. Mechanism for hepato-protective action of Liangxue Huayu Recipe (LHR): Blocked of mitochondrial cytochrome c release and caspase activation. J. Ethnopharm. 2013, 148, 851–860. [Google Scholar] [CrossRef]
- Yin, Q.H.; Yan, F.X.; Zu, X.Y.; Wu, Y.H.; Wu, X.P.; Liao, M.C.; Deng, S.W.; Yin, L.L.; Zhuang, Y.Z. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechology 2012, 64, 43–51. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, P.; Xiao, W.; Fan, L.Q.; Wang, F.J.; Li, S.X.; Liu, J.W. A novel human derived cell-penetrating peptide in drug delivery. Mol. Biol. Rep. 2011, 38, 2649–2656. [Google Scholar] [CrossRef]
- Wu, W.; Yao, D.F.; Wang, Y.L.; Qiu, L.W.; Sai, Q.L.; Yang, J.L.; Yao, N.H.; Li, S.S.; Bian, Y.Z.; Wang, A.W.; et al. Suppression of human hepatoma (HepG2) cell growth by nuclear factor-kappaB/p65 specific siRNA. Tumor Biol. 2010, 31, 605–611. [Google Scholar] [CrossRef]
- Wang, C.D.; Wang, H.B.; Cheng, R.; Gou, S.M.; Liu, T. Effect of Target-directed Regulation of Uncoupling Protein-2 Gene Expression on Ischemia-reperfusion Injury of Hepatocytes. J. Huazhong Univ. Sci. Technol. 2008, 28, 558–563. [Google Scholar] [CrossRef]
- Uemura, D.; Ohwaki, H.; Hirata, Y. Isolation and structures of 20-deoxyingenol, new diterpene, derivatives, and ingenol derivative obtained from kansui. Tetrahedron Lett. 1974, 29, 2527–2528. [Google Scholar] [CrossRef]
- Pan, D.J.; Qi, H.C.; Chang, J.J.; Lee, T.T.Y.; Chen, Y.P.; Hsu, H.Y.; McPhail, D.R.; McPhail, A.T.; Lee, K.H. Kansuiphorin C and D, cytotoxic diterpenes from Euphorbia kansui. Phytochemistry 1991, 30, 1018–1020. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, N.L.; Yao, X.S.; Miyata, S.; Kitanaka, S. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus (2). Chem. Pharm. Bull. 2003, 51, 935–941. [Google Scholar] [CrossRef]
- Pan, Q.; Min, Z.D. Studies on ingenol-type diterpene esters in root tuber of Euphorbia kansui. Chin. Tradit. Herb Drugs 2003, 34, 489–492. (In Chinese) [Google Scholar]
- Li, C.F. Studies of the Constituents of the Processed Roots of Euphorbia kansui T. N. Liou ex T. N. Wang. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, May 2006. [Google Scholar]
- Wang, Y.B.; Li, Y.Y.; Wang, H.B.; Qin, G.W. Chemical constituents from the roots of Euphorbia kansui. Chin. J. Nat. Med. 2007, 5, 182–185. [Google Scholar]
- Nunomura, S.; Kitanaka, S.; Ra, C. 3-O-(2,3-Dimethylbutanoyl)-13-O-decanoylingenol from Euphorbia kansui suppresses IgE-mediated mast cell activation. Biol. Pharm. Bull. 2006, 29, 286–290. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhong, J.M.; Ye, S.Q.; Ni, Z.Y.; Miao, X.Q.; Mo, Y.K.; Li, Z.L. Screening of Epstein-Barr virus early antigen expression inducers from Chinese medicinal herbs and plants. Biomed. Environ. Sci. 1994, 7, 50–55. [Google Scholar]
- Shi, J.X.; Li, Z.X.; Nitoda, T.; Izumi, M.; Kanzaki, H.; Baba, N.; Kawazu, K.; Nakajima, S. Three antinematodal diterpenes from Euphorbia kansui. Biosci. Biotechnol. Biochem. 2007, 71, 1086–1089. [Google Scholar] [CrossRef]
- Ogasawara, J.; Wstanaba-Fukunaga, R.; Adachi, M. Lethal effect of the anti-Fas antibody in mice. Nature 1993, 364, 806–809. [Google Scholar] [CrossRef]
- Kass, C.E. Mitochondrial involvement in drug-induced hepatic injury. Chem. Biol. Interact. 2006, 163, 145–159. [Google Scholar] [CrossRef]
- Green, D.R. Apoptotic pathways: The roads to run. Cell 1998, 94, 695–698. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1308–1312. [Google Scholar]
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mark, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol 2008, 9, 532–542. [Google Scholar] [CrossRef]
- Bouchier-Hayes, L.; Munoz-Pinedo, C.; Connell, S.; Green, D.R. Measuring apoptosis at the single cell level. Methods 2008, 44, 222–228. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, W.C.; Liu, Q.S.; Hu, J.J.; Liu, G.T.; Du, G.H. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur. J. Pharmacol. 2008, 591, 73–79. [Google Scholar] [CrossRef]
- Lv, L.; Zhou, Z.X.; Huang, X.Z.; Zhao, Y.P.; Zhang, L.; Shi, Y.X.; Sun, M.L.; Zhang, J.W. Inhibition of peptidyl-prolyl cis/trans isomerase Pin1 induces cell cycle arrest and apoptosis in vascular smooth muscle cells. Apoptosis 2010, 15, 41–54. [Google Scholar] [CrossRef]
- Guan, L.Y.; Han, B.S.; Li, Z.S.; Hua, F.Y.; Huang, F.; Wei, W.; Yang, Y.; Caimin, X. Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis 2009, 14, 218–225. [Google Scholar] [CrossRef]
- Palacios, C.; Yerbes, R.; Lopez-Rivas, A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006, 66, 8858–8869. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, X.Y.; Ding, A.W.; Tang, Y.P.; Duan, J.A.; Shang, E.X.; Shen, X.C. LC-DAD-ESI-MS-MS separation and chemical characterization of the inflammatory fraction of the roots of Euphorbia kansui. Chromatographia 2009, 70, 5–6. [Google Scholar]
- Shu, X.Y.; Yu, L.; Tang, Y.P.; Zhang, L.; Ding, A.W.; Luo, D.; Duan, J.A.; Shen, X.C. Bioassay-guided separation of the proinflammatory constituents from the roots of Euphorbia kansui. J. Nat. Med. 2010, 64, 98–103. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.Q.; Song, F.R.; Liu, S.Y. Optimization of Preparing Process Condition of Kansui Roots by Electrospray Ionization Mass Spectrometry. J. Chin. Mass Spectrom. Soc. 2010, 31, 72–78. [Google Scholar]
- Fago, A.; Mathews, A.J.; Moens, L.; Dewilde, S.; Brittain, T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006, 580, 4884–4888. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, X.; Zhang, L.; Guo, J.; Cao, Y.; Shang, E.; Tang, Y.; Ding, A.; Duan, J.-A. Processing of Kansui Roots Stir-Baked with Vinegar Reduces Kansui-Induced Hepatocyte Cytotoxicity by Decreasing the Contents of Toxic Terpenoids and Regulating the Cell Apoptosis Pathway. Molecules 2014, 19, 7237-7254. https://doi.org/10.3390/molecules19067237
Yan X, Zhang L, Guo J, Cao Y, Shang E, Tang Y, Ding A, Duan J-A. Processing of Kansui Roots Stir-Baked with Vinegar Reduces Kansui-Induced Hepatocyte Cytotoxicity by Decreasing the Contents of Toxic Terpenoids and Regulating the Cell Apoptosis Pathway. Molecules. 2014; 19(6):7237-7254. https://doi.org/10.3390/molecules19067237
Chicago/Turabian StyleYan, Xiaojing, Li Zhang, Jianming Guo, Yudan Cao, Erxin Shang, Yuping Tang, Anwei Ding, and Jin-Ao Duan. 2014. "Processing of Kansui Roots Stir-Baked with Vinegar Reduces Kansui-Induced Hepatocyte Cytotoxicity by Decreasing the Contents of Toxic Terpenoids and Regulating the Cell Apoptosis Pathway" Molecules 19, no. 6: 7237-7254. https://doi.org/10.3390/molecules19067237