Study on the Synthesis, Biological Activity and Spectroscopy of Naphthalimide-Diamine Conjugates
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

2.2. Cytotoxic Effects
| Compd. | IC50(µM) | ||
|---|---|---|---|
| K562 | HCT116 | 7701 | |
| 7a | 48.76 ± 3.93 | 19.33 ± 4.39 | >50 |
| 7b | 25.60 ± 3.74 | 13.93 ± 2.68 | 33.89 ± 4.32 |
| 7c | 38.75 ± 1.76 | 9.89 ±1.11 | >50 |
| 7d | 19.71 ± 7.23 | 8.95 ± 3.87 | 8.04 ±3.35 |
| 7e | 22.36 ± 1.21 | 9.52 ± 3.31 | 22.16 ± 0.67 |
| 7f | 18.87 ± 1.97 | 5.45 ± 1.71 | 19.69 ± 3.42 |
| 7g | 14.03 ± 3.32 | 13.88 ± 2.54 | 11.97 ± 4.07 |
| 7h | 15.89 ± 1.53 | 16.21 ± 4.28 | 18.78 ± 4.94 |
| 7i | 41.35 ± 1.76 | 10.97 ± 1.71 | >50 |
| 7j | 12.56 ± 0.81 | 16.44 ±1.75 | 13.13 ± 3.94 |
| 7k | 15.89 ± 1.83 | 9.95 ± 1.81 | 14.70 ± 3.91 |
| Amonafide | 10.10 ± 2.59 | 6.86 ± 1.89 | 20.29 ± 2.43 |
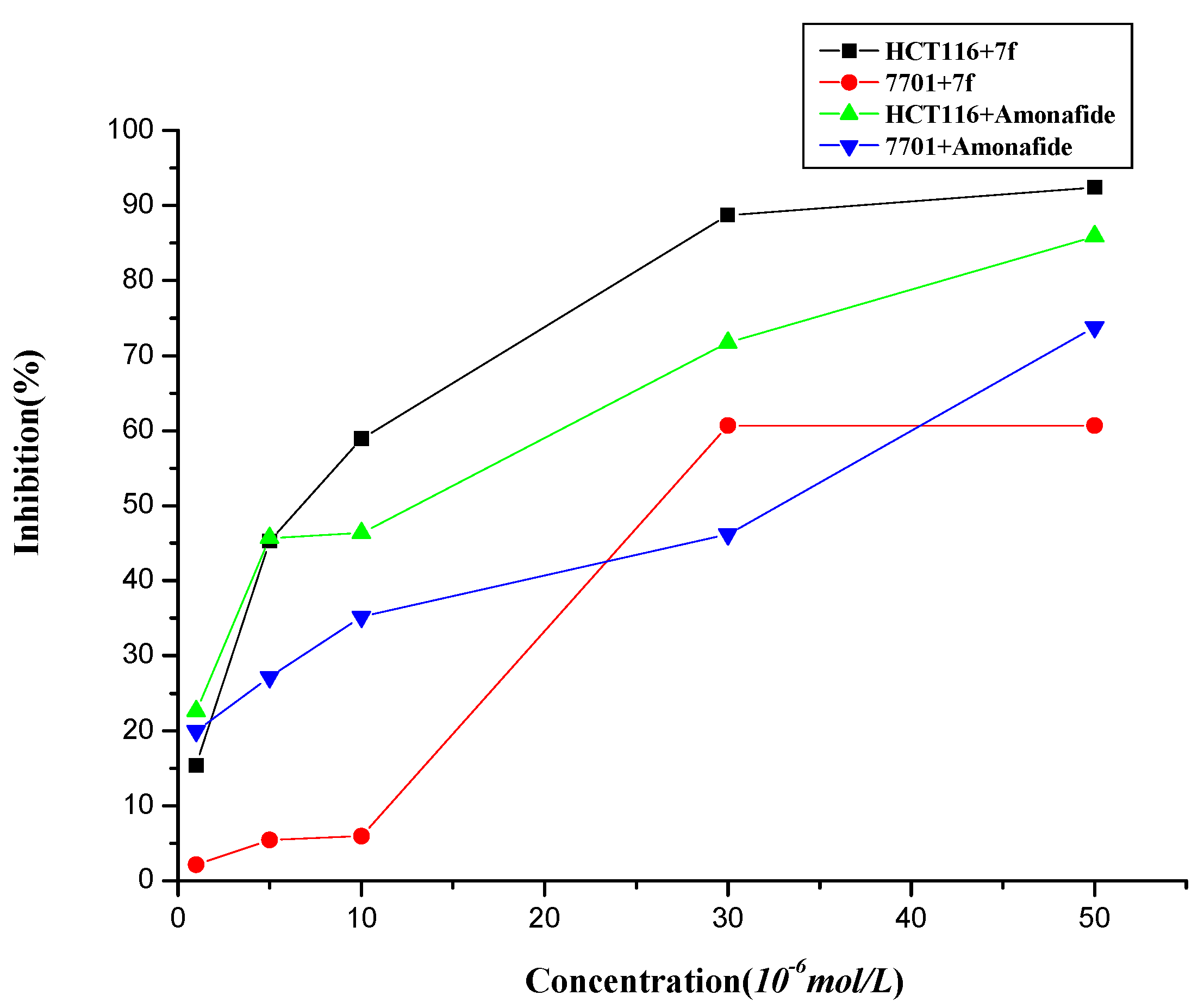
2.3. Cell Cycle Analysis and Apoptosis


2.4. UV Spectroscopy



2.5. Fluorescence Spectroscopy
2.5.1. Fluorescence Quenching


2.5.2. Fluorescence Quenching Mechanism

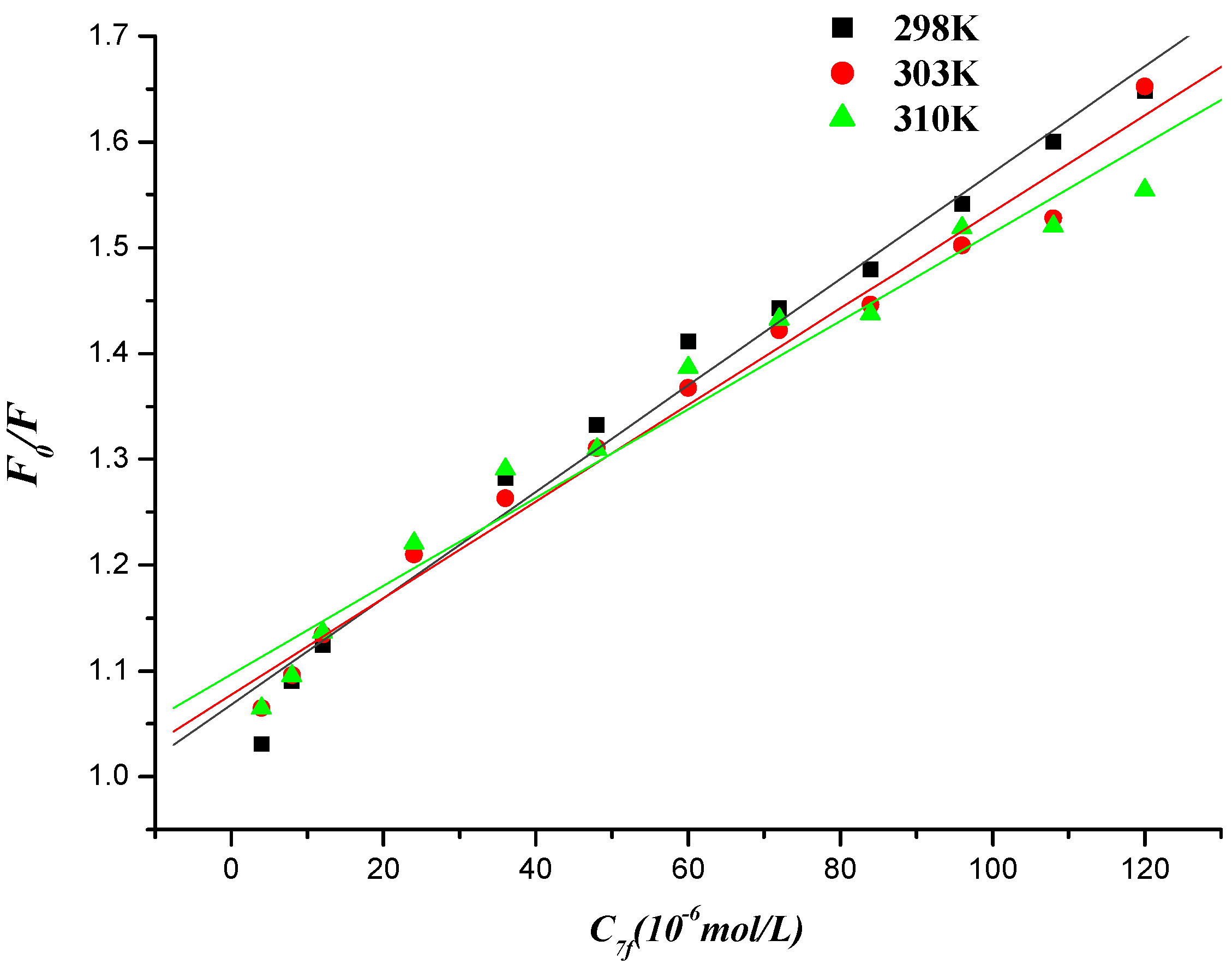
| T/K | KSV/(L∙mol−1) | Kq/(L∙mol−1) | R |
|---|---|---|---|
| 298 | 5.567 × 103 | 5.567 × 1011 | 0.9956 |
| 303 | 4.789 × 103 | 4.789 × 1011 | 0.9987 |
| 310 | 3.651 × 103 | 3.651 × 1011 | 0.9945 |
| T/K | KSV/(L∙mol−1) | Kq/(L∙mol−1) | R |
|---|---|---|---|
| 298 | 5.028 × 103 | 5.028 × 1011 | 0.9907 |
| 303 | 4.564 × 103 | 4.654 × 1011 | 0.9922 |
| 310 | 4.176 × 103 | 4.176 × 1011 | 0.9809 |
2.5.3. Interaction Mode between Compounds and DNA

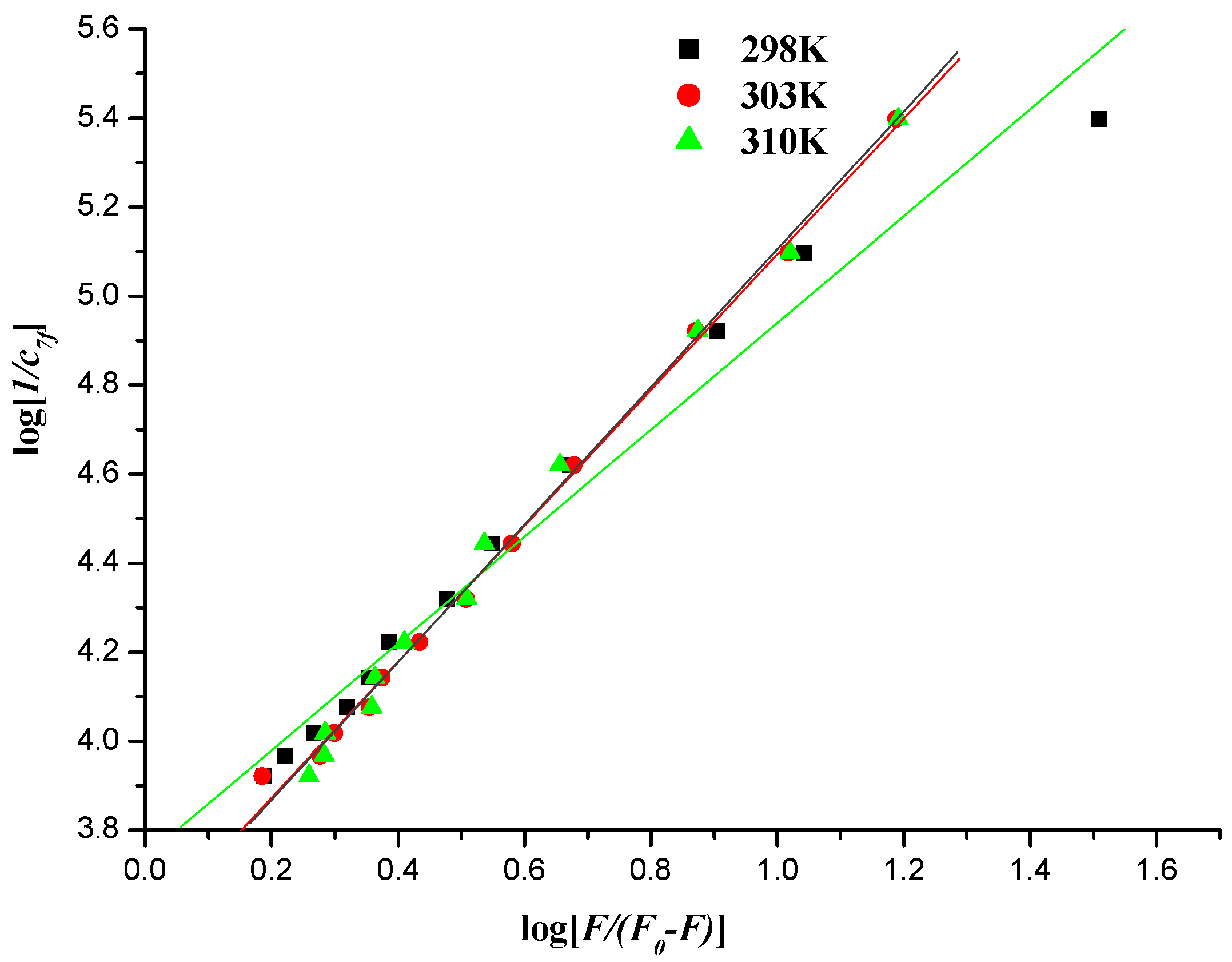
| T/K | Kb/(kJ∙mol−1) | ∆G°/(kJ∙mol−1) | ∆H°/(kJ∙mol−1) | ∆S°/(kJ∙mol−1) | R |
|---|---|---|---|---|---|
| 298 | 6.059 × 103 | −21.578 | −32.983 | −0.0383 | 0.9897 |
| 303 | 4.864 × 103 | −21.387 | −32.983 | −0.0383 | 0.9965 |
| 310 | 3.462 × 103 | −21.004 | −32.983 | −0.0383 | 0.9923 |
| T/K | Kb/(kJ∙mol−1) | ∆G°/(kJ∙mol−1) | ∆H°/(kJ∙mol−1) | ∆S°/(kJ∙mol−1) | R |
|---|---|---|---|---|---|
| 298 | 5.482 × 103 | −21.330 | −59.229 | −0.127 | 0.9884 |
| 303 | 3.695 × 103 | −21.172 | −59.229 | −0.127 | 0.9982 |
| 310 | 3.611 × 103 | −21.112 | −59.229 | −0.127 | 0.9975 |
2.5.4. Iodide Quenching Studies

2.5.5. Effect of Ionic Intensity on the Compound 7f and DNA Interaction

3. Experimental Section
3.1. General Information
3.2. Synthesis of Naphthalimide-Diamine Conjugates
3.3. Biological Materials and Methods
3.3.1. Cell Culture
3.3.2. MTT Assay
3.4. Cell Cycle Analysis and Apoptosis
3.4.1. Cell Cycle Analysis
3.4.2. Apoptosis
3.5. Spectroscopy Measurement
3.5.1. Apparatus
3.5.2. UV–Vis Measurements
3.5.3. Fluorescence Measurement
3.5.3.1. Interaction of Compound 7f with DNA
3.5.3.2. Interaction of Compound 7f with DNA-EB Complex
3.5.3.3. Iodide Quenching
3.5.3.4. Effect of Ionic Intensity on The Interaction between Compound 7f and DNA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braña, M.F.; Sanz, A.M.; Castellano, J.M.; Roldán, C.M.; Roldán, C. Synthesis and cytostatic activity of benz[de]isoquinoline-1,3-diones, structure-activity relationships. Eur. J. Med. Chem. Chim. Ther. 1981, 16, 207–212. [Google Scholar]
- Sami, S.M.; Dorr, R.T.; Alberts, D.S.; Remers, W.A. 2-Substituted 1,2-dihydro- 3H- dibenz [de,h] isoquinoline-1, 3-diones. A new class of antitumor agent. J. Med. Chem. 1993, 36, 765–770. [Google Scholar] [CrossRef]
- Baily, C.; Braña, M.F.; Waring, M.J. Sequence-selective intercalation of antitumor bisnaphthalimides into DNA. Evident for an approach via the major groove. Eur. J. Biochem. 1996, 240, 195–208. [Google Scholar] [CrossRef]
- Braña, M.F.; Castellano, J.M.; Morán, M.; Pérez de Vega, M.J.; Perron, D.; Conlon, D.; Bousquet, P.F.; Romerdahl, C.A.; Robinson, S.P. Bis naphthalimides 3: Synthesis and antitumor activity of N,N'-bis[2-(1,8-naphthalimido)-ethyl]alkanedimines. Anticancer Drug Des. 1996, 11, 297–309. [Google Scholar]
- Rosell, R.; Carles, J.; Abad, A.; Ribelles, N.; Barnadas, A.; Benavides, A.; Martin, M. Phase I study of mitonafide in 120 h continuous infusion in non-small cell lung cancer. Investig. New Drugs 1992, 10, 171–175. [Google Scholar] [CrossRef]
- Costanza, M.E.; Berry, D.; Henderson, I.C.; Ratain, M.J.; Wu, K.; Shapiro, C.; Duggan, D.; kalra, J.; Berkowitz, I.; Lyss, A.P. Amonafide: An active agent in the treatment of previously untreated advanced breast cancer—a cancer and leukemia group B study (CALGB 8642). Clin. Cancer Res. 1995, 1, 699–704. [Google Scholar]
- Sami, S.M.; Dorr, R.T.; Sólyom, A.M.; Alberts, D.S.; Remers, W.A. Amino-substituted 2-[2'-(dimethyl-amino)ethyl 1,2-dihydro-3H-dibenz[de,h] isoquinoline-1,3-diones. Synthesis, antitumor activity and quantitative structure- activity relation-ship. J. Med. Chem. 1995, 38, 983–993. [Google Scholar] [CrossRef]
- Thompson, J.; Pratt, C.B.; Stewart, C.F.; Avery, L.; Bowman, L.; Zamboni, W.C.; Pappo, A. Phase I study of DMP 840 in pediatric patients with refractory solid tumors. Investig. New Drugs 1998, 16, 45–49. [Google Scholar]
- Nitiss, J.L.; Zhou, J.; Rose, A.; Hsiung, Y.; Gale, K.C.; Osheroff, N. The bis(naphthalimide) DMP-840 causes cytotoxicity by its action against eukaryotic topoisomerase II. Biochemistry 1998, 37, 3078–3085. [Google Scholar] [CrossRef]
- Bousquet, P.F.; Braña, M.F.; Conlon, D.; Fitzgerald, K.M.; Perron, D.; Cocchiaro, C.; Miller, R.; Moran, M.; George, J.; Qian, X.D.; et al. Preclinical evaluation of LU 79553: A novel bisnaphthalimide with potent antitumor activity. Cancer Res. 1995, 55, 1176–1180. [Google Scholar]
- Diaz-Rubio, E.; Martin, M.; López-Vega, J.M.; Casado, A.; Benavides, A. Phase I study of mitonafide with a 3-day administration schedule: Early interruption due to severe central nervous system toxity. Investig. New Drugs 1994, 12, 277–281. [Google Scholar] [CrossRef]
- Braña, M.F.; Domínguez, G.; Sáez, B.; Romerdahl, C.; Robinson, S.; Barlozzari, T. Synthesis and antitumour activity of new dendritic polyamines-(imide-DNA-intercalator) conjugates: Potent Lck inhibitors. Eur. J. Med. Chem. 2002, 37, 541–551. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; Ramos, A.; Dominguez, M.T.; Pozuelo, J.M.; Abradelo, C.; Rey-Stolle, M.F.; Yuste, M.; Carrasco, C.; Bailly, C. Synthesis, biological evaluation and DNA binding properties of novel mono and bisnaphthalimides. Org. Biomol. Chem. 2003, 1, 648–654. [Google Scholar] [CrossRef]
- Van Quaquebeke, E.; Mahieu, T.; Dumont, P.; Dewelle, J.; Ribaucour, F.; Simon, G.; Sauvage, S.; Gaussin, J.F.; Tuti, J.; El Yazidi, M.; et al. 2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de] isoquinolin-5-yl} car-bamoyl) acetamide (UN BS3157), a novel nonhematotoxic naphthalimide derivative with potent antitumor activity. J. Med. Chem. 2007, 50, 4122–4134. [Google Scholar]
- Chen, Z.; Liang, X.; Zhang, H.Y.; Xie, H.; Liu, J.W.; Xu, Y.F.; Zhu, W.P.; Wang, Y.; Wang, X.; Tan, S.Y.; et al. A new class of naphthalimide-based antitumor agents that inhibit topoisomerase II and induce lysosomal membrane permeabilization and apoptosis. J. Med. Chem. 2010, 53, 2589–2600. [Google Scholar] [CrossRef]
- Sk, U.H.; Prakasha Gowda, A.S.; Crampsie, M.A.; Yun, J.K.; Spratt, T.E.; Amin, S.; Sharma, A.K. Development of novel naphthalimide derivatives and their evaluation as potential melanoma therapeutics. Eur. J. Med. Chem. 2011, 46, 3331–3338. [Google Scholar] [CrossRef]
- Roy, S.S.; Ghosh, P.; Sk, U.H.; Chakraborty, P.; Biswas, J.; Mandal, S.; Bhattacharjee, A.; Bhattacharya, S. Naphthalimide based novel organo-selenocyanates: Finding less toxic forms of selenium that would retain protective efficacy. Bioorg. Med. Chem. Lett. 2010, 21, 6951–6955. [Google Scholar]
- Filosa, R.; Peduto, A.; Micco, S.D.; Caprariis, P.D.; Festa, M.; Petrella, A.; Capranico, G.; Bifulco, G. Molecular modeling studies, synthesis and biological activity of a series of novel bisnaphthalimides and their development as new DNA topoisomerase II inhibitors. Bioorg. Med. Chem. 2009, 17, 13–24. [Google Scholar]
- Kamal, A.; Ramu, R.; Tekumalla, V.; Khanna, G.B.; Barkume, M.S.; Juvekar, A.S.; Zingde, S.M. Remarkable DNA binding affinity and potential anticancer activity of pyrrolo[2,1-c][1,4]benzodiazepine-naphthalimide conjugates linked through piperazine side-armed alkane spacers. Bioorg. Med. Chem. 2008, 16, 7218–7224. [Google Scholar] [CrossRef]
- Machado, K.E.; Oliveira, K.N.; Santos-Bubniak, L.; Licínio, M.A.; Nunes, R.J.; Santos-Silva, M.C. Evaluation of apoptotic effect of cyclic imide derivatives on murine B16F10 melanoma cells. Bioorg. Med. Chem. 2011, 19, 6285–6291. [Google Scholar] [CrossRef]
- Minarini, A.; Milelli, A.; Tumiatti, V.; Ferruzzi, L.; Marton, M.R.; Turrini, E.; Hrelia, P.; Fimognari, C. Design, synthesis and biological evaluation of new naphtalene diimides bearing isothiocyanate functionality. Eur. J. Med. Chem. 2012, 48, 124–131. [Google Scholar] [CrossRef]
- Barron, G.A.; Bermano, G.; Gordon, A.; Kong Thoo Lin, P. Synthesis, cytotoxicity and DNA-binding of novel bisnaphthalimidopropyl derivatives in breast cancer MDA-MB-231 cells. Eur. J. Med. Chem. 2010, 45, 1430–1437. [Google Scholar] [CrossRef]
- Banerjee, S.; Kitchen, J.A.; Bright, S.A.; O’Brien, J.E.; Williams, D.C.; Kelly, J.M.; Gunnlaugsson, T. Synthesis, spectroscopic and biological studies of a fluorescent Pt(II) (terpy) based 1,8-naphthalimide conjugate as a DNA targeting agent. Chem. Commun. 2013, 49, 8522–8524. [Google Scholar] [CrossRef]
- Banerjee, S.; Kitchen, J.A.; Gunnlaugsson, T.; Kelly, J.M. The effect of the 4-amino functionality on the photophysical and DNA binding properties of alkyl-pyridinium derived 1,8-naphthalimides. Org. Biomol. Chem. 2013, 11, 5642–5655. [Google Scholar] [CrossRef]
- Banerjee, S.; Kitchen, J.A.; Gunnlaugsson, T.; Kelly, J.M. Synthesis and photophysical evaluation of a pyridinium 4-amino-1,8-naphthalimide derivative that upon intercalation displays preference for AT-rich double-stranded DNA. Org. Biomol. Chem. 2012, 10, 3033–3043. [Google Scholar] [CrossRef]
- Wang, C.J.; Delcros, J.G.; Cannon, L.; Konate, F.; Carias, H.; Bigger staff, J.; Gardner, R.A.; Phanstiel, O., IV. Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J. Med. Chem. 2003, 46, 5129–5138. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Xie, S.Q.; Du, Y.W.; Ma, Y.F.; Zhao, J.; Gao, W.Y.; Wang, C.J. Synthesis, cytotoxicity and apoptosis of naphthalimide polyamine conjugates as antitumor agents. Eur. J. Med. Chem. 2009, 44, 393–399. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Xie, S.Q.; Mei, Z.H.; Zhao, J.; Gao, W.Y.; Wang, C.J. Conjugation of substituted naphthalimides to polyamines as cytotoxic agents targeting the Akt/mTOR signal pathway. Org. Biomol. Chem. 2009, 7, 4551–4560. [Google Scholar] [CrossRef]
- Yang, L.H.; Li, W.; Tian, Z.Y.; Zhao, J.; Wang, C.J. Mononaphthalimide spermidine conjugate induces cell proliferation inhibition and apoptosis in HeLa cells. Toxicol. In Vitro 2011, 52, 882–889. [Google Scholar]
- Pasternack, R.F.; Gibbs, E.J.; Villafranca, J.J. Interactions of water souble porphyrins with Z-poly (dG-dC). Biochemistry 1983, 22, 2406–2414. [Google Scholar]
- Xie, S.Q.; Cheng, P.F.; Liu, G.C.; Ma, Y.F.; Zhao, J.; Chehtane, M.; Khaled, A.R.; Phanstiel, O., IV; Wang, C.J. Synthesis and bioevaluation of N-(arylalkyl)-homospermidine conjugates. Bioorg. Med. Chem. Lett. 2007, 17, 4471–4475. [Google Scholar]
- Wang, C.J.; Delcros, J.G.; Bigger staff, J.; Phanstiel, O., IV. Molecular requirements for targeting the polyamine transport system. Synthesis and biological evaluation of polyamine-anthracene conjugates. J. Med. Chem. 2003, 46, 2672–2682. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar]
- Tian, Z.Y.; Su, L.P.; Xie, S.Q.; Zhao, J.; Wang, C.J. Study on synthesis, biological activitity and fluorescence spectroscopy of naphthalimide-polyamine conjugates. Chin. J. Org. Chem. 2013, 33, 1514–1521. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, M.; Yang, F.; Chen, Y.; Miao, Z.H.; Qian, X.H.; Xu, Y.F.; Qin, Y.X.; Luo, H.B.; Shen, X.; et al. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Mol. Cancer Ther. 2007, 6, 484–495. [Google Scholar] [CrossRef]
- Long, E.C.; Barton, J.K. On demonstrating DNA intercalation. Acc. Chem. Res. 1990, 23, 271–273. [Google Scholar] [CrossRef]
- Tysoe, S.A.; Morgan, R.J.; Baker, A.D.; Strekas, T.C. Spectroscopic investigation of differential binding modes of Δ- and Λ-Ru(bpy)2(ppz)2+ with calf thymus DNA. J. Phys. Chem. 1993, 97, 1707–1711. [Google Scholar] [CrossRef]
- Li, N.; Ma, Y.; Yang, C.; Gong, L.P.; Yang, X.H. Interaction of anticancer drug mitoxantrone with DNA analyzed by electrochemical and spectroscopic methods. Biophys. Chem. 2005, 116, 199–205. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltametry. J. Photoch. Photobio. B: Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Ghosh, K.S.; Bera, R.; Dasgupta, S. Studies on the interaction of diacetylcurcumin with calf thymus-DNA. Chem. Phys. 2008, 351, 163–169. [Google Scholar] [CrossRef]
- Ghaderi, M.; Bathaie, S.Z.; Saboury, A.A.; Sharghi, H.; Tangestaninejad, S. Interaction of an Fe derivative of TMAP (Fe (TMAP) OAc) with DNA in comparison with free-base TMAP. Int. J. Biol. Macromol. 2007, 41, 173–179. [Google Scholar] [CrossRef]
- Hassanien, M.M.; Abou-EI-Sherbini, K.S. Synthesis and characterisation of morin-functionalised silica gel for the enrichment of some precious metal ions. Talanta 2006, 68, 1550–1559. [Google Scholar] [CrossRef]
- Selim, M.; Chowdhury, S.R.; Mukherjea, K.K. DNA binding and nuclease activity of a one-dimensional heterometallic nitrosyl complex. Int. J. Biol. Macromol. 2007, 41, 579–583. [Google Scholar] [CrossRef]
- Lin, H.; Lan, J.F.; Guan, M.; Sheng, F.L.; Zhang, H.X. Spectroscopic investigation of interaction between mangiferin and bovine serum albumin. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2009, 73, 936–941. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Zhao, R.M.; Dong, J.X.; Qu, S.S. Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. J. Photochem. Photobiol. A: Chem. 2006, 179, 324–329. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.H.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modeling. Biochim. Biophys. Acta 2002, 1594, 84–99. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, S.; Yin, C.; Liu, L.S.; Hu, C.M.; Zhao, Y.Y.; Ye, Y.X.; Hu, X.Y.; Fan, J. Synthesis and spectroscopic characterization of 4-butoxyethoxy-N- octadecyl-1,8- naphthalimide as a new fluorescent probe for the determination of proteins. Bioorg. Med. Chem. Lett. 2011, 21, 3798–3804. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1999; pp. 237–265. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; pp. 97–155. [Google Scholar]
- Hu, Y.J.; Liu, Y.; Zhang, L.X.; Zhao, R.M.; Qu, S.S. Study of interaction between colchicines and bovine serum albumin by fluorescence quenching method. J. Mol. Struct. 2005, 750, 174–178. [Google Scholar] [CrossRef]
- Feng, Q.; Li, N.Q.; Jiang, Y.Y. Electrochemical studies of porphyrin interacting with DNA and determination of DNA. Anal.Chim. Acta 1997, 344, 97–104. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Xiao, X.H. Investigation of the interaction between Berberine and human serum albumin. Biomacromolecules 2009, 10, 517–521. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Takenaka, S.; Ihara, T.; Takagi, M. Bis-9-acridinyl derivative containing a viologen linker chain: Electrochemically active intercalator for reversible labelling of DNA. J. Chem. Soc. Chem. Commun. 1990, 23, 1485–1487. [Google Scholar] [CrossRef]
- Qiao, C.Y.; Bi, S.Y.; Sun, Y.; Song, D.Q.; Zhang, H.Q.; Zhou, W.H. Study of interactions of anthraquinones with DNA using ethidium bromide as a fluorescence probe. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2008, 70, 136–143. [Google Scholar] [CrossRef]
- Zhang, G.W.; Hu, X.; Zhao, N.; Li, W.B.; He, L. Studies on the interaction of aminocarb with calf thymus DNA by spectroscopic methods. Pestic. Biochem. Phys. 2010, 98, 206–212. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tian, Z.-Y.; Li, J.-H.; Li, Q.; Zang, F.-L.; Zhao, Z.-H.; Wang, C.-J. Study on the Synthesis, Biological Activity and Spectroscopy of Naphthalimide-Diamine Conjugates. Molecules 2014, 19, 7646-7668. https://doi.org/10.3390/molecules19067646
Tian Z-Y, Li J-H, Li Q, Zang F-L, Zhao Z-H, Wang C-J. Study on the Synthesis, Biological Activity and Spectroscopy of Naphthalimide-Diamine Conjugates. Molecules. 2014; 19(6):7646-7668. https://doi.org/10.3390/molecules19067646
Chicago/Turabian StyleTian, Zhi-Yong, Jing-Hua Li, Qian Li, Feng-Lei Zang, Zhong-Hua Zhao, and Chao-Jie Wang. 2014. "Study on the Synthesis, Biological Activity and Spectroscopy of Naphthalimide-Diamine Conjugates" Molecules 19, no. 6: 7646-7668. https://doi.org/10.3390/molecules19067646




