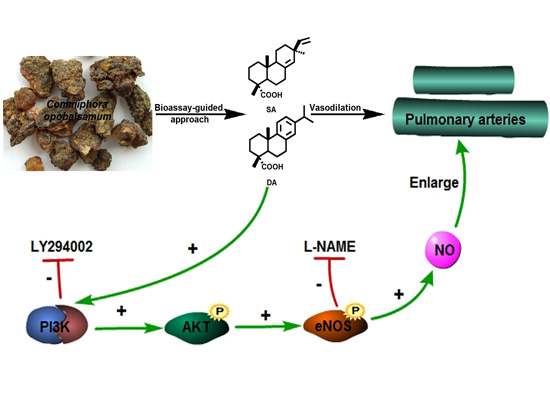
Dehydroabietic Acid Isolated from Commiphora opobalsamum Causes Endothelium-Dependent Relaxation of Pulmonary Artery via PI3K/Akt-eNOS Signaling Pathway
Abstract
:1. Introduction

2. Results and Discussion
2.1. Bioassay-Guided Isolation

2.2. Elucidation of Compounds’ Structures
 : +2.0° (c 0.10 EtOH)} and 2{
: +2.0° (c 0.10 EtOH)} and 2{  : +63.0° (c 0.10 EtOH)} were in accordance with previous reported data [12]. By comparing their 1H- and 13C-NMR data with that of previous studies [13,14], compounds 1 and 2 were identified as SA and DA, respectively (Figure 1).
: +63.0° (c 0.10 EtOH)} were in accordance with previous reported data [12]. By comparing their 1H- and 13C-NMR data with that of previous studies [13,14], compounds 1 and 2 were identified as SA and DA, respectively (Figure 1).2.3. Effect of DA and SA on Pulmonary Arteries

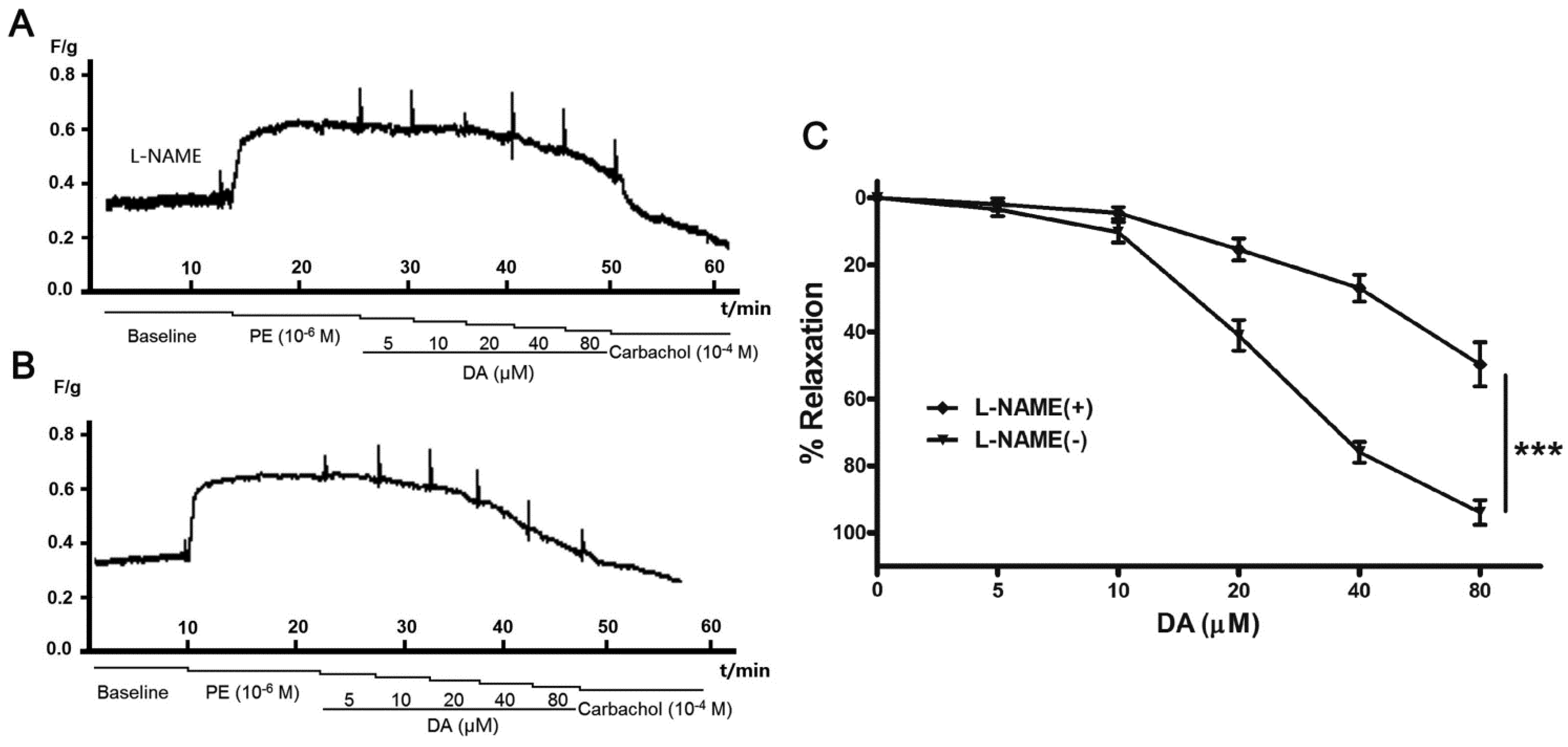
2.4. Effect of Endothelium and L-NAME on DA-Induced Vasorelaxation

2.5. DA Promotes NO Release of PAECs

2.6. Role of the PI3K/Akt Pathway in DA-Induced Relaxation


3. Experimental Section
3.1. General Information
3.2. Materials and Reagents
3.3. Extraction and Isolation
3.4. Vessel Rings Preparation
3.5. Effect of All the Parts on Pulmonary Artery Rings
3.6. Culture of Pulmonary Artery Endothelial Cells (PAECs)
3.7. Measurement of NO Production in PAECs
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Montani, D.; Gunther, S.; Dorfmuller, P. Pulmonary arterial hypertension. Orphanet J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef]
- Benza, R.; Miller, D.; Gomberg-Maitland, M.; Frantz, R.; Foreman, A.; Coffey, C.; Frost, A.; Barst, R.; Badesch, D.; Elliott, C.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Al-Yahya, M.; Al-Meshal, L.; Mossa, J.; Al-Badr, A.; Tariq, M. Saudi Plants; King Saud University Press: Riyadh, Saudi Arabia, 1990. [Google Scholar]
- Shen, T.; Wan, W.; Yuan, H.; Kong, F.; Guo, H.; Fan, P.; Lou, H. Secondary metabolites from Commiphora opobalsamum and their antiproliferative effect on human prostate cancer cells. Phytochemistry 2007, 68, 1331–1337. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.; Amin, R. Effect of aqueous extract of Commiphora opobalsamum on blood pressure and heart rate in rats. J. Ethnopharmacol. 1997, 57, 219–222. [Google Scholar] [CrossRef]
- Al-Howiriny, T.; Al-Sohaibani, M.; Al-Said, M.; Al-Yahya, M.; El-Tahir, K.; Rafatullah, S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. J. Ethnopharmacol. 2005, 98, 287–294. [Google Scholar] [CrossRef]
- Al-Howiriny, T.; Al-Sohaibani, M.; Al-Said, M.; Al-Yahya, M.; El-Tahir, K.; Rafatullah, S. Hepatoprotective properties of Commiphora opobalsamum (“Balessan”), a traditional medicinal plant of Saudi Arabia. Drugs Exp. Clin. Res. 2004, 30, 213–220. [Google Scholar]
- Senejoux, F.; Demougeot, C.; Kerram, P.; Aisa, H.; Berthelot, A.; Bevalot, F.; Girard-Thernier, C. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae). Fitoterapia 2012, 83, 377–382. [Google Scholar] [CrossRef]
- Hipolito, U.; Rodrigues, G.; Lunardi, C.; Bonaventura, D.; Ambrosio, S.; de Oliveira, A.; Bendhack, L.; da Costa, F.; Tirapelli, C. Mechanisms underlying the vasorelaxant action of the pimarane-ent-8(14),15-pimaradien-3β-ol in the isolated rat aorta. Eur. J. Pharmacol. 2009, 616, 183–191. [Google Scholar] [CrossRef]
- Ambrosio, S.; Tirapelli, C.; Bonaventura, D.; de Oliveira, A.; da Costa, F. Pimarane diterpene from Viguiera arenaria (Asteraceae) inhibit rat carotid contraction. Fitoterapia 2002, 73, 484–489. [Google Scholar] [CrossRef]
- Tirapelli, C.; dos Anjos Neto Filho, M.; Bonaventura, D.; Melo, M.; Ambrosio, S.; de Oliveira, A.; Bendhack, L.; da Costa, F. Pimaradienoic acid inhibits vascular contraction and induces hypotension in normotensive rats. J. Pharm. Pharmacol. 2008, 60, 453–459. [Google Scholar] [CrossRef]
- Su, S.; Duan, J.; Tang, Y.; Zhang, X.; Yu, L.; Jiang, F.; Zhou, W.; Luo, D.; Ding, A. Isolation and biological activities of neomyrrhaol and other terpenes from the resin of Commiphora myrrha. Planta Med. 2009, 75, 351–355. [Google Scholar]
- Fang, J.; Tsai, W.; Chebg, Y. Serratene triterpenes from Pinus armandii Bark. Phytochemistry 1991, 30, 1333–1335. [Google Scholar] [CrossRef]
- Cheung, H.; Toshio, M.; Mark, P. Further acidic constituents and neutral components of Pinus massoniana Resin. Tetrahedron 1993, 40, 7903–7907. [Google Scholar] [CrossRef]
- Ohwada, T.; Nonomura, T.; Maki, K.; Sakamoto, K. Dehydroabietic acid derivatives as a novel scaffold for large-conductance calcium-activated K+ channel openers. Bioorg. Med. Chem. Lett. 2003, 13, 3971–3974. [Google Scholar] [CrossRef]
- Rios, M.; Lopez-Martinez, S.; Lopez-Vallejo, F.; Medina-Franco, J.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarrete-Vazquez, G.; Hidalgo-Figueroa, S.; Hernandez-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef]
- Scalera, F.; Martens-Lobenhoffer, J.; Tager, M.; Bukowska, A.; Lendeckel, U.; Bode-Boger, S. Effect of l-arginine on asymmetric dimethylarginine (ADMA) or homocysteine-accelerated endothelial cell aging. Biochem. Biophys. Res. Commun. 2006, 345, 1075–1082. [Google Scholar] [CrossRef]
- Koh, S.; Kang, M.; Kim, T.; Park, H.; Park, C.; Seong, Y.; Seong, H. Endothelium-dependent vasodilatory and hypotensive effects of Crotalaria sessiliflora L. in rats. Biol. Pharm. Bull. 2007, 30, 48–53. [Google Scholar] [CrossRef]
- Kwan, C.; Zhang, W.; Sim, S.; Deyama, T.; Nishibe, S. Vascular effects of Siberian ginseng (Eleutherococcus senticosus): Endothelium-dependent NO- and EDHF-mediated relaxation depending on vessel size. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 473–480. [Google Scholar] [CrossRef]
- Vasques, C.; Cortes, S.; Silva, M.; de Medeiros, I. Muscarinic agonist properties of the hydrobutanol extract from aerial parts of Waltheria viscosissima St. Hil. (Sterculiaceae) in rats. Phytother. Res. 1999, 13, 312–317. [Google Scholar] [CrossRef]
- Jiang, F.; Li, C.G.; Rand, M.J. Mechanisms of nitric oxide-independent relaxations induced by carbachol and acetylcholine in rat isolated renal arteries. Br. J. Pharmacol. 2000, 130, 1191–1200. [Google Scholar] [CrossRef]
- Kim, K.; Moriarty, K.; Bender, J. Vascular cell signaling by membrane estrogen receptors. Steroids 2008, 73, 864–869. [Google Scholar] [CrossRef]
- Ndiaye, M.; Chataigneau, M.; Lobysheva, I.; Chataigneau, T.; Schini-Kerth, V. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005, 19, 455–457. [Google Scholar]
- Jin, S.N.; Wen, J.F.; Wang, T.T.; Kang, D.G.; Lee, H.S.; Cho, K.W. Vasodilatory effects of ethanol extract of Radix Paeoniae Rubra and its mechanism of action in the rat aorta. J. Ethnopharmacol. 2012, 142, 188–193. [Google Scholar] [CrossRef]
- Rattmann, Y.; Anselm, E.; Kim, J.; Dal-Ros, S.; Auger, C.; Miguel, O.; Santos, A.; Chataigneau, T.; Schini-Kerth, V. Natural product extract of Dicksonia sellowiana induces endothelium-dependent relaxations by a redox-sensitive Src- and Akt-dependent activation of eNOS in porcine coronary arteries. J. Vasc. Res. 2012, 49, 284–298. [Google Scholar] [CrossRef]
- Luo, Z.; Fujio, Y.; Kureishi, Y.; Rudic, R.D.; Daumerie, G.; Fulton, D.; Sessa, W.C.; Walsh, K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide- dependent vasomotor activity in vivo. J. Clin. Investig. 2000, 106, 493–499. [Google Scholar] [CrossRef]
- Bai, Y.; Lu, P.; Han, C.; Yu, C.; Chen, M.; He, F.; Yi, D.; Wu, L. Hydroxysafflor Yellow A (HSYA) from Flowers of Carthamus tinctorius L. and Its Vasodilatation Effects on Pulmonary Artery. Molecules 2012, 17, 14918–14927. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Li, Y.; Leu, C.; Guo, L.; Zheng, X.; Zhu, D. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur. J. Pharmacol. 2008, 588, 9–17. [Google Scholar] [CrossRef]
- Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Fluorescent indicators for imaging nitric oxide production. Angew. Chem. Int. Ed. Engl. 1999, 38, 3209–3212. [Google Scholar] [CrossRef]
- Sheng, B.; Gong, K.; Niu, Y.; Liu, L.; Yan, Y.; Lu, G.; Zhang, L.; Hu, M.; Zhao, N.; Zhang, X.; et al. Inhibition of γ-secretase activity reduces Aβ production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis:Implications for the treatment of Alzheimer’s disease. Free Radic. Biol. Med. 2009, 46, 1362–1375. [Google Scholar] [CrossRef]
- Yao, L.; Lu, P.; Li, Y.; Yang, L.; Feng, H.; Huang, Y.; Zhang, D.; Chen, J.; Zhu, D. Osthole relaxes pulmonary arteries through endothelial phosphatidylinositol 3-kinase/Akt-eNOS-NO signaling pathway in rats. Eur. J. Pharmacol. 2013, 699, 23–32. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the dehydroabietic acid (DA) and sandaracopimaric acid (SA) are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, W.; Dong, X.; Xie, N.; Zhou, C.; Fan, Y.; Chen, G.; Wang, Y.; Wei, T.; Zhu, D. Dehydroabietic Acid Isolated from Commiphora opobalsamum Causes Endothelium-Dependent Relaxation of Pulmonary Artery via PI3K/Akt-eNOS Signaling Pathway. Molecules 2014, 19, 8503-8517. https://doi.org/10.3390/molecules19068503
Gao W, Dong X, Xie N, Zhou C, Fan Y, Chen G, Wang Y, Wei T, Zhu D. Dehydroabietic Acid Isolated from Commiphora opobalsamum Causes Endothelium-Dependent Relaxation of Pulmonary Artery via PI3K/Akt-eNOS Signaling Pathway. Molecules. 2014; 19(6):8503-8517. https://doi.org/10.3390/molecules19068503
Chicago/Turabian StyleGao, Wenyan, Xiaoyan Dong, Nan Xie, Chunlan Zhou, Yuhua Fan, Guoyou Chen, Yanming Wang, Taiming Wei, and Daling Zhu. 2014. "Dehydroabietic Acid Isolated from Commiphora opobalsamum Causes Endothelium-Dependent Relaxation of Pulmonary Artery via PI3K/Akt-eNOS Signaling Pathway" Molecules 19, no. 6: 8503-8517. https://doi.org/10.3390/molecules19068503