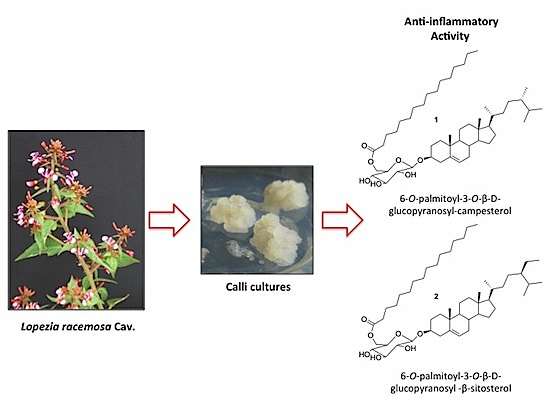
Production of the Anti-Inflammatory Compound 6-O-Palmitoyl-3-O-β-D-glucopyranosylcampesterol by Callus Cultures of Lopezia racemosa Cav. (Onagraceae)
Abstract
:1. Introduction
2. Results and Discussion
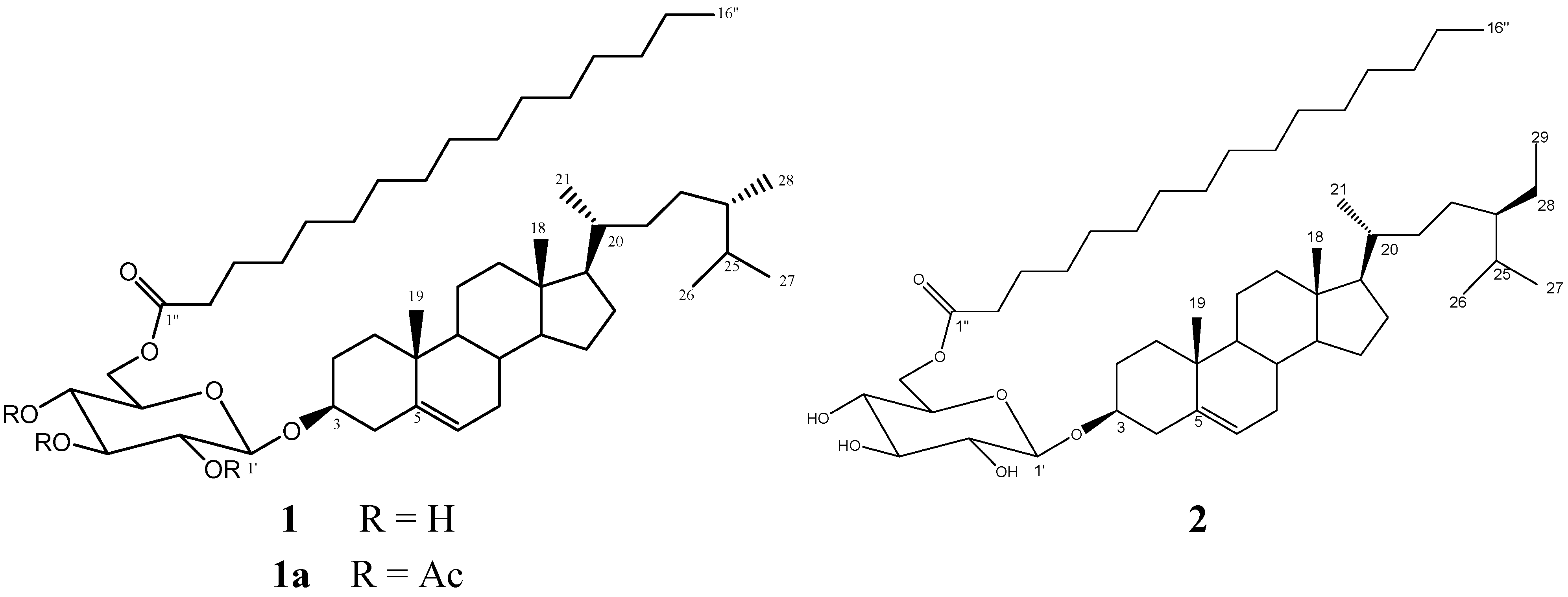
2.1. Bioassay-Guided Chromatographic Purification and Characterization of Compound 1
| Sample | Percentage of Anti-Inflammatory Activity (1 mg/ear) | IC50 (mg/ear) |
|---|---|---|
| SMM a | 16.81 ± 0.96 | >1 |
| SMD b | 58.40 ± 1.63 | 0.93 ± 0.06 |
| SMH c | 48.74 ± 1.01 | >1 |
| LR50A | 35.30 ± 1.44 | >1 |
| LR50B | 86.26 ± 1.89 | ND e |
| LR50C | −30.72 ± 1.49 | >1 |
| LR50D | 19.47 ± 2.21 | >1 |
| LR50F | 11.26 ± 2.57 | >1 |
| LR50G | 33.78 ± 2.54 | >1 |
| LR50H | 31.30 ± 1.48 | >1 |
| 1 d | 57.14 ± 1.81 | 0.45 ± 0.08 |
| INDOMETHACIN | 91.35 ± 0.87 | 0.14 ± 0.09 |
2.2. Callus Production
| Treatments (mg/L) | Photoperiod | Darkness | ||||
|---|---|---|---|---|---|---|
| Leaf | Stem Node | Hypocotyl | Leaf | Stem Node | Hypocotyl | |
| 1) NAA 0.5 | L b | S b | H b | L b | S ad | H b |
| 2) NAA 1.0/BAP 0.5 | L a | S a | H a | L a | S a | H a |
| 3) NAA 2.0/BAP 0.5 | L b | S b | H a | L a | S b | H ad |
| 4) NAA 4.0/BAP 0.5 | L b | S b | H a | L a | S b | H a |
| 5) 2,4-D 0.5 | L a | S ad | H a | L a | S ad | H a |
| 6) 2,4-D 1.0/BAP 0.5 | L ad | S a | H a | L a | S a | H a |
| 7) 2,4-D 2.0/BAP 0.5 | L a | S a | H a | L a | S a | H a |
| 8) 2,4-D 4.0/BAP 0.5 | L a | S a | H a | L a | S a | H a |
| 9) BAP 0.5 | L c | S c | H c | L c | S c | H c |
| 10) Without PGR | L c | S c | H c | L c | S c | H c |
2.3. Callus Lines Selection
| Callus Line or Source of 1 | Biomass DW(g) | DCM Extract (mg) | µg of 1 in DCM Extract ± SD | µg of 1/g Biomass ± SD |
|---|---|---|---|---|
| SN0.5K | 2.25 | 22.55 | 38.61 ± 3.16 | 17.17 ± 1.38 |
| HN2.0/B0.5K | 3.34 | 21.45 | 153.21 ± 5.13 | 45.80 ± 1.53 |
| SD0.5P | 2.61 | 37.41 | 289.27 ± 50.64 | 110.83 ± 19.40 |
| SD0.5K | 3.07 | 36.85 | 493.09 ± 14.26 | 160.61 ± 4.61 |
| LD1.0/B0.5P | 3.91 | 73.15 | 678.54 ± 90.08 | 173.98 ± 23.09 |
| Wild Plants | 3170.01 | 31,304.01 | 4,935,000.60 ± 3377.22 | 1556.80 ± 103.92 |
| Seedlings | 10.81 | 124.21 | 23,070.60 ± 1292.98 | 2130.13 ± 120.02 |
2.4. Compound 1 Production

3. Experimental
3.1. Chemical Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation of 1 from Wild Plant
3.4. Compound 1a
 +0.076 (c 0.1, CHCl3); IR (Nujol) νmax 1756.8 cm−1; 1H-NMR (CDCl3, 400 MHz): δH 5.28 (1H, d, J = 4.8 Hz, H-6), 5.13 (1H, t, J = 10.0 Hz, H-3'), 4.05 (1H, d, J = 7.6 Hz, H-1'), 4.97 (1H, t, J = 9.2 Hz, H-4'), 4.88, (1H, t, J = 9.6 Hz, H-2'), 4.15 (1H, dd, J = 5.2, 12.0 Hz, H-6'a), 4.05 (1H, dd, J = 2.1, 12.0 Hz, H-6'b), 3.61 (1H, ddd, J = 2.4, 5.6, 10.0 Hz, H-5'), 3.41 (1H, m, H-3), 2.25 (2H, m, H-2''), 2.16 (2H, m, H-4), 1.98 (3H, s, CH3CO), 1.95 (3H, s, CH3CO), 1.93 (3H, s, CH3CO), 1.94 (2H, m, H-12), 1.8 (2H, m, H-2), 1.60 (1H, m, H-25), 1.58 (2H, m, H-3''), 1.57 (2H, m, H-15), 1.44 (2H, m, H-11), 1.40 (1H, m, H-8), 1.33 (1H, m, H-20), 1.26 (2H, m, H-22), 1.25 (24H, m, aliphatic chain), 1.2 (2H, m, H-7), 1.18 (2H, m, H-28), 1.16 (2H, m, H-16), 1.06 (1H, m, H-17), 0.98 (2H, m, H-1), 0.94 (1H, m, H-14), 0.91 (3H, s, H-19), 0.86 (1H, m, H-24), 0.84 (3H, d, J = 6.4 Hz, H-9, H-21), 0.80 (3H, t, J = 6.8 Hz, H-29), 0.77 (3H, t, J = 6.0 Hz, H-16''), 0.75 (6H, s, H-26, H-27), 0.60 (3H, m, H-18); 13C NMR (CDCl3, 100 MHz): δC 173.6 (C-1''), 169.5 (COCH3), 169.56 (COCH3), 170.5 (COCH3), 140.4 (C-5), 122.3 (C-6), 99.7 (C-1'), 80.2 (C-3), 73.0 (C-3'), 71.8 (C-5'), 71.6 (C-2'), 68.8 (C-4'), 62.1 (C-6'), 56.8 (C-14), 56.2 (C-17), 50.2 (C-9), 45.9 (C-24), 42.4 (C-13), 39.8 (C-12), 39.0 (C-4), 37.3 (C-1), 36.8 (C-10), 36.2 (C-20), 34.3 (C-2''), 34.0 (C-22), 32.1 (C-7), 32.0 (C-8), 29.2 (C-25), 28.3 (C-2), 26.1 (C-16), 24.9 (C-3''), 24.4 (C-15), 21.1 (C-11), 20.89 (CH3CO), 20.82 (CH3CO), 20.7 (CH3CO), 19.9 (C-27), 19.4 (C-26), 19.1 (C-19), 18.9 (C-21), 14.3 (C-16''), 12.2 (C-28), 11.9 (C-18) ; HRESIMS m/z 949.6738 (calcd for C56H94O10Na, 949.6744).
+0.076 (c 0.1, CHCl3); IR (Nujol) νmax 1756.8 cm−1; 1H-NMR (CDCl3, 400 MHz): δH 5.28 (1H, d, J = 4.8 Hz, H-6), 5.13 (1H, t, J = 10.0 Hz, H-3'), 4.05 (1H, d, J = 7.6 Hz, H-1'), 4.97 (1H, t, J = 9.2 Hz, H-4'), 4.88, (1H, t, J = 9.6 Hz, H-2'), 4.15 (1H, dd, J = 5.2, 12.0 Hz, H-6'a), 4.05 (1H, dd, J = 2.1, 12.0 Hz, H-6'b), 3.61 (1H, ddd, J = 2.4, 5.6, 10.0 Hz, H-5'), 3.41 (1H, m, H-3), 2.25 (2H, m, H-2''), 2.16 (2H, m, H-4), 1.98 (3H, s, CH3CO), 1.95 (3H, s, CH3CO), 1.93 (3H, s, CH3CO), 1.94 (2H, m, H-12), 1.8 (2H, m, H-2), 1.60 (1H, m, H-25), 1.58 (2H, m, H-3''), 1.57 (2H, m, H-15), 1.44 (2H, m, H-11), 1.40 (1H, m, H-8), 1.33 (1H, m, H-20), 1.26 (2H, m, H-22), 1.25 (24H, m, aliphatic chain), 1.2 (2H, m, H-7), 1.18 (2H, m, H-28), 1.16 (2H, m, H-16), 1.06 (1H, m, H-17), 0.98 (2H, m, H-1), 0.94 (1H, m, H-14), 0.91 (3H, s, H-19), 0.86 (1H, m, H-24), 0.84 (3H, d, J = 6.4 Hz, H-9, H-21), 0.80 (3H, t, J = 6.8 Hz, H-29), 0.77 (3H, t, J = 6.0 Hz, H-16''), 0.75 (6H, s, H-26, H-27), 0.60 (3H, m, H-18); 13C NMR (CDCl3, 100 MHz): δC 173.6 (C-1''), 169.5 (COCH3), 169.56 (COCH3), 170.5 (COCH3), 140.4 (C-5), 122.3 (C-6), 99.7 (C-1'), 80.2 (C-3), 73.0 (C-3'), 71.8 (C-5'), 71.6 (C-2'), 68.8 (C-4'), 62.1 (C-6'), 56.8 (C-14), 56.2 (C-17), 50.2 (C-9), 45.9 (C-24), 42.4 (C-13), 39.8 (C-12), 39.0 (C-4), 37.3 (C-1), 36.8 (C-10), 36.2 (C-20), 34.3 (C-2''), 34.0 (C-22), 32.1 (C-7), 32.0 (C-8), 29.2 (C-25), 28.3 (C-2), 26.1 (C-16), 24.9 (C-3''), 24.4 (C-15), 21.1 (C-11), 20.89 (CH3CO), 20.82 (CH3CO), 20.7 (CH3CO), 19.9 (C-27), 19.4 (C-26), 19.1 (C-19), 18.9 (C-21), 14.3 (C-16''), 12.2 (C-28), 11.9 (C-18) ; HRESIMS m/z 949.6738 (calcd for C56H94O10Na, 949.6744).3.5. Compound 2
3.6. Acid Hydrolysis of Compound 1
3.7. Seed Germination and Obtaining Axenic Seedling
3.8. Callus Induction
3.9. Calli Selection
3.10. Extraction of 1 from Selected Callus Lines
3.11. Identification and Quantification of Compound 1 in Selected Calli Lines
3.12. Model of Mice Ear Inflammation Induced with TPA

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mulabagal, V.; Hsin-Sheng, T. Plant Cell Cultures: An Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. Int. J. App. Sci. Eng. 2004, 2, 29–48. [Google Scholar]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Inze, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant. Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural sources as potential anti-cancer agents: A review. Inter. J. Phytomed. 2011, 3, 9–26. [Google Scholar]
- Rodzi, R.; Cheah, Y.L.; Ooi1, K.K.; Othman, F.; Mohtarrudin, N.; Tohid, S.F.; Suhaili, Z.; Zakaria, Z.A. Chemopreventive potential of methanol extract of Dicranopteris linearis leaf on DMBA/croton oil-induced mouse skin carcinogenesis. Afr. J. Pharm. Pharmacol. 2013, 7, 2484–2498. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharm. 2011, 133, 945–972. [Google Scholar] [CrossRef]
- Cruz Paredes, C.; Bolívar Balbás, P.; Gómez-Velasco, A.; Hernández, L.R.; Juárez, Z.N.; Sánchez Arreola, E.; Bach, H. Antimicrobial, Antiparasitic, Anti-Inflammatory, and Cytotoxic Activities of Lopezia racemosa. Sci. World J. 2013, 2013, 237438:1–237438:6. [Google Scholar]
- Qadeer, G.; Rama, N.H; Hill, R.A; Garduño-Ramírez, M.L. Synthesis and anti-inflammatory activity of fluorinated isocoumarins and 3,4 dihydroisocoumarins. J. Fluorine Chem. 2007, 128, 641–646. [Google Scholar] [CrossRef]
- Rubnov, S.; Kashman, Y.; Rabinowitz, R.; Schlesinger, M. Suppresors of Cancer Cell Proliferation from Fig (Ficus. carica) Resin: Isolation and structure elucidation. J. Nat. Prod. 2001, 64, 993–996. [Google Scholar] [CrossRef]
- Frenkel, M.; Marsh, K.N. Spectral Data for Steroids; TRC Data Series; TRC: College Station, TX, USA, 1994; p. 530. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 1 are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salinas, R.; Arellano-García, J.; Perea-Arango, I.; Álvarez, L.; Garduño-Ramírez, M.L.; Marquina, S.; Zamilpa, A.; Castillo-España, P. Production of the Anti-Inflammatory Compound 6-O-Palmitoyl-3-O-β-D-glucopyranosylcampesterol by Callus Cultures of Lopezia racemosa Cav. (Onagraceae). Molecules 2014, 19, 8679-8690. https://doi.org/10.3390/molecules19068679
Salinas R, Arellano-García J, Perea-Arango I, Álvarez L, Garduño-Ramírez ML, Marquina S, Zamilpa A, Castillo-España P. Production of the Anti-Inflammatory Compound 6-O-Palmitoyl-3-O-β-D-glucopyranosylcampesterol by Callus Cultures of Lopezia racemosa Cav. (Onagraceae). Molecules. 2014; 19(6):8679-8690. https://doi.org/10.3390/molecules19068679
Chicago/Turabian StyleSalinas, Roberta, Jesús Arellano-García, Irene Perea-Arango, Laura Álvarez, María Luisa Garduño-Ramírez, Silvia Marquina, Alejandro Zamilpa, and Patricia Castillo-España. 2014. "Production of the Anti-Inflammatory Compound 6-O-Palmitoyl-3-O-β-D-glucopyranosylcampesterol by Callus Cultures of Lopezia racemosa Cav. (Onagraceae)" Molecules 19, no. 6: 8679-8690. https://doi.org/10.3390/molecules19068679