Effects of Icariin on Reproductive Functions in Male Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Icariin on Body Weight

2.2. Effects of Icariin on Organ Coefficients

2.3. Effects of Icariin on Testicular Morphology

2.4. Icariin-Induced Changes in Epididymal Sperm Count
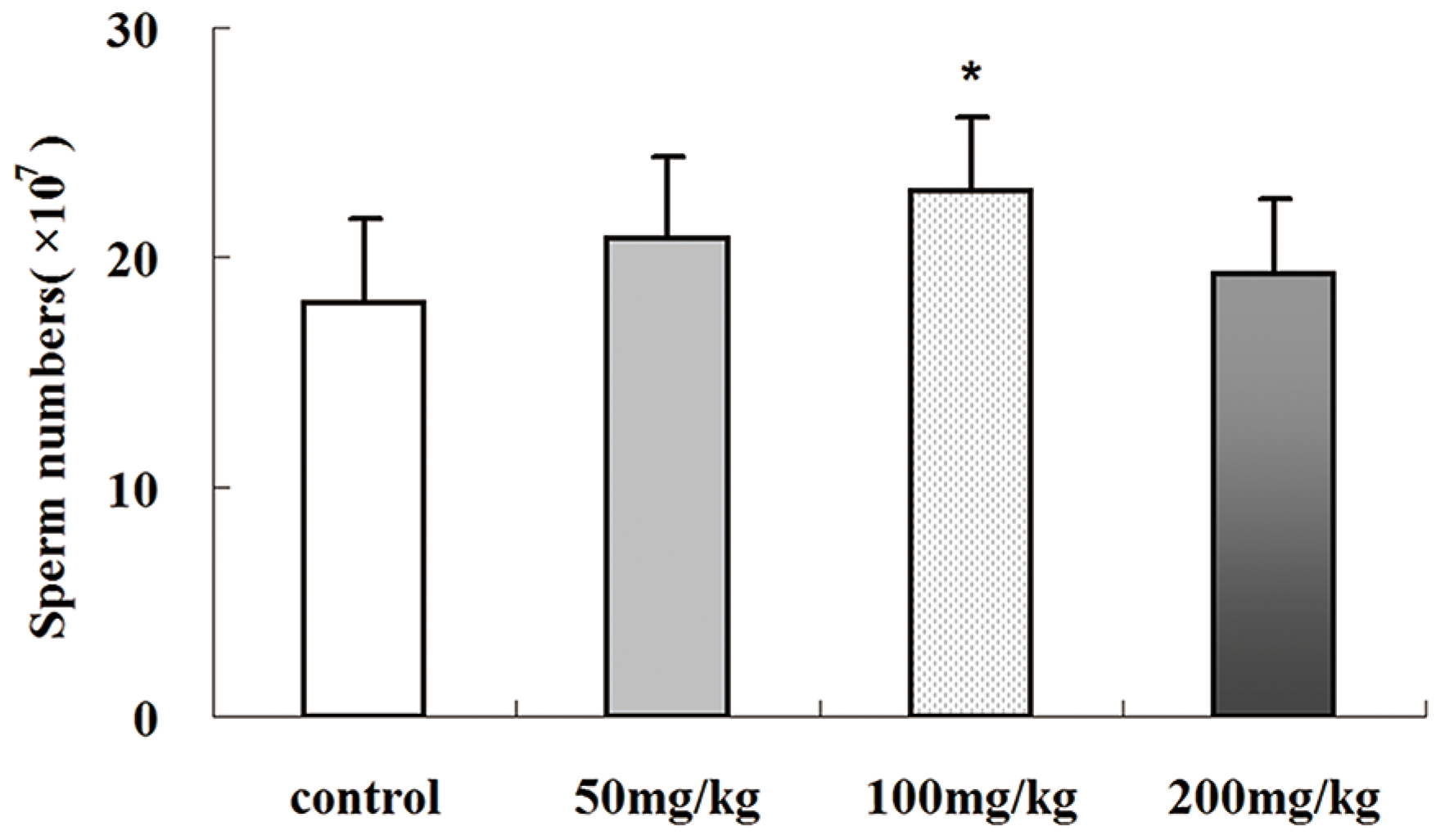
2.5. Effects of Icariin on Serum Testosterone Production

2.6. Effects of Icariin on mRNA Expression Levels of Luteinizing Hormone Receptor (LHR) and Steroidogenic Genes

2.7. Effects of Icariin on the mRNA Expression Levels of Several Sertoli Cell-Specific Genes
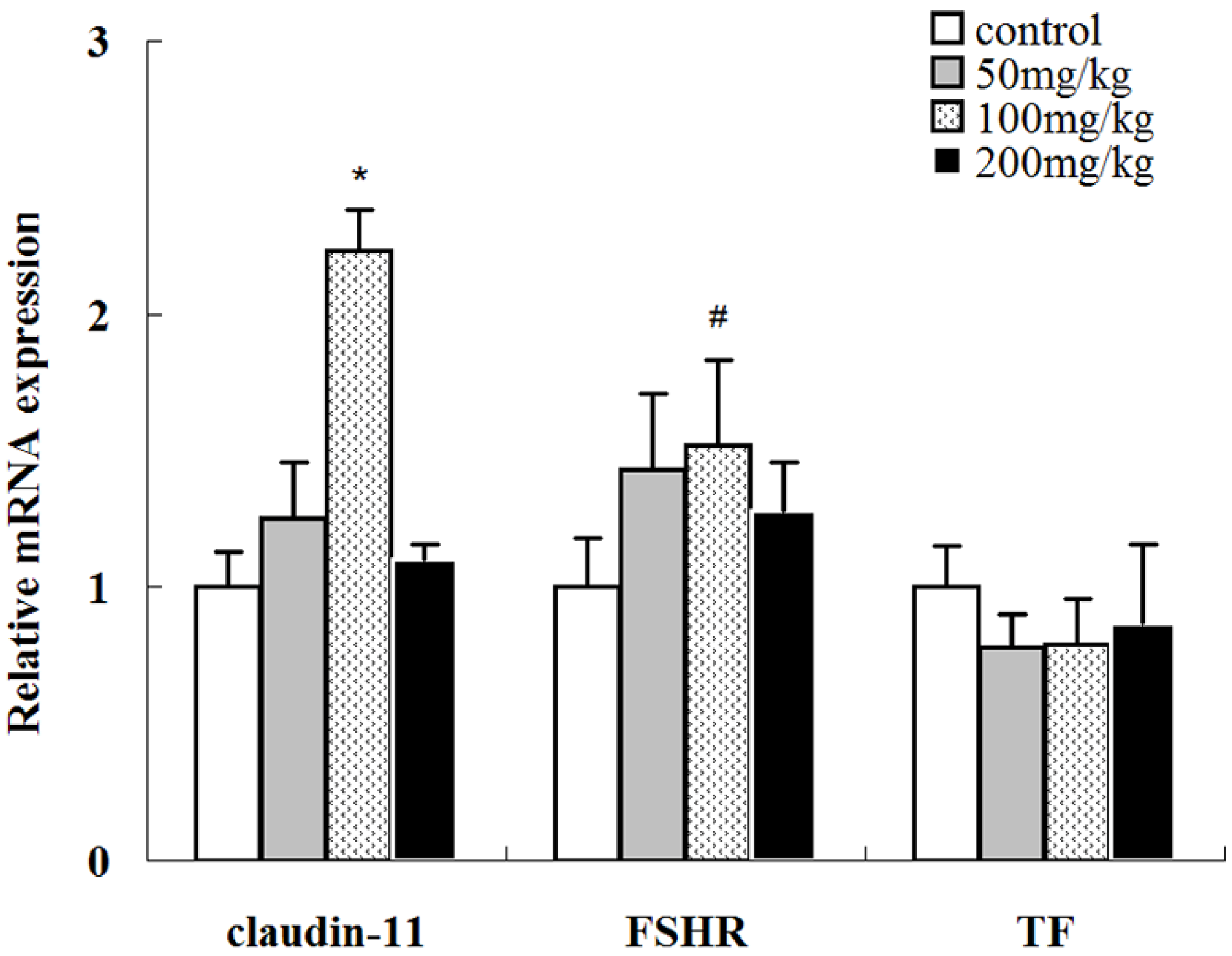
2.8. Effects of Icariin on SOD Activities and MDA Levels in Rat Testis
| Groups | SOD (U/mg) | MDA (nmol/ mg) |
|---|---|---|
| Control | 96.1 ± 10.84 | 3.47 ± 0.3 |
| 50 mg/kg 100 mg/kg | 115.83 ± 17.45 * 123.53 ± 25.65 * | 3.1 ± 0.32 # 3.55 ± 0.33 |
| 200 mg/kg | 103.73 ± 20.21 | 4.12 ± 0.47 * |
3. Experimental
3.1. Materials

3.2. Animal Experiments
3.3. Relative Weights of Reproductive Organs
3.4. Epididymal Sperm Count
3.5. Histopathological Examination
3.6. Detection of Serum Testosterone
3.7. Real-Time PCR
| Gene | Sequence | Length | References |
|---|---|---|---|
| Actin | ACGTTGACATCCGTAAAGAC | 200 bp | |
| GAAGGTGGACAGTGAGGC | |||
| PBR | ACACTGGTCAGCTGGCTCTGAA | 175 bp | [37] |
| CAGGCCAGGTAAGGATACAGCAA | |||
| StAR | GGGCATACTCAACAACCAG | 111 bp | [38] |
| ACCTCCAGTCGGAACACC | |||
| LHR | CATTCAATGGGACGACTCTA | 130 bp | [38] |
| GCCTGCAATTTGGTGGA | |||
| P450SCC | AGTATCCGTGATGTGGGG | 125 bp | [38] |
| CATACAGTGTCGCCTTTTCT | |||
| Cyp17a1 | GCAGAGTTACTTGCCCTTCGG | 142 bp | [38] |
| CAGGCGGGGCAGTTGTTTAT | |||
| 3β-HSD1 | TGTGCCAGCCTTCATCTAC | 145 bp | [38] |
| CTTCTCGGCCATCCTTTT | |||
| Claudin-11 | GCTTCGTGGGTTGGAT | 82 bp | |
| CAGGTGGGGATGGTGTA | |||
| TF | GCTGTGGCCAGTTTCTTCTC | 163 bp | [39] |
| CCACATCTCCACCTCCATCT | |||
| FSHR | GGCCAGGTCAACATACCGCTTG | 162 bp |
3.8. Determination of SOD Activity and MDA Level
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine 2010, 17, 414–423. [Google Scholar] [CrossRef]
- Kang, H.K.; Choi, Y.H.; Kwon, H.; Lee, S.B.; Kim, D.H.; Sung, C.K.; Park, Y.I.; Dong, M.S. Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components: In vitro and in vivo studies. Food Chem. Toxicol. 2012, 50, 2751–2759. [Google Scholar] [CrossRef]
- Shindel, A.W.; Xin, Z.C.; Lin, G.; Fandel, T.M.; Huang, Y.C.; Banie, L.; Breyer, B.N.; Garcia, M.M.; Lin, C.S.; Lue, T.F. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J. Sex. Med. 2010, 7, 1518–1528. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Lin, S.; Lu, H.; Xu, J. Icariin enhances the healing of rapid palatal expansion induced root resorption in rats. Phytomedicine 2012, 19, 1035–1041. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, J.; Pu, J.; Zhao, L.; Lu, Z.; Hu, J.; Yu, Q.; Wang, Y.; Xie, Y.; Li, G. Icariin induces apoptosis in mouse MLTC-10 Leydig tumor cells through activation of the mitochondrial pathway and down-regulation of the expression of piwil4. Int. J. Oncol. 2011, 39, 973–980. [Google Scholar]
- Wu, B.; Chen, Y.; Huang, J.; Ning, Y.; Bian, Q.; Shan, Y.; Cai, W.; Zhang, X.; Shen, Z. Icariin improves cognitive deficits and activates quiescent neural stem cells in aging rats. J. Ethnopharmacol. 2012, 142, 746–753. [Google Scholar] [CrossRef]
- Zhang, D.W.; Cheng, Y.; Wang, N.L.; Zhang, J.C.; Yang, M.S.; Yao, X.S. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine 2008, 15, 55–61. [Google Scholar] [CrossRef]
- Fan, J.J.; Cao, L.G.; Wu, T.; Wang, D.X.; Jin, D.; Jiang, S.; Zhang, Z.Y.; Bi, L.; Pei, G.X. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules 2011, 16, 10123–10133. [Google Scholar] [CrossRef]
- Low, W.Y.; Tan, H.M. Asian traditional medicine for erectile dysfunction. J. Men’s Health Gender 2007, 4, 245–250. [Google Scholar] [CrossRef]
- Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N.; Tesakova, S.V.; Makarov, V.G.; Tikhonov, V.P. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J. Ethnopharmacol. 2007, 114, 412–416. [Google Scholar] [CrossRef]
- Liu, T.; Xin, H.; Li, W.R.; Zhou, F.; Li, G.Y.; Gong, Y.Q.; Gao, Z.Z.; Qin, X.C.; Cui, W.S.; Shindel, A.W.; et al. Effects of icariin on improving erectile function in streptozotocin-induced diabetic rats. J. Sex. Med. 2011, 8, 2761–2772. [Google Scholar] [CrossRef]
- Chiu, J.H.; Chen, K.K.; Chien, T.M.; Chiou, W.F.; Chen, C.C.; Wang, J.Y.; Lui, W.Y.; Wu, C.W. Epimedium brevicornum maxim extract relaxes rabbit corpus cavernosum through multitargets on nitric oxide/cyclic guanosine monophosphate signaling pathway. Int. J. Impot. Res. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Dal Cero, E.; Belluti, F.; Matera, R.; Zironi, E.; Pagliuca, G.; Bosisio, E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J. Nat. Prod. 2008, 71, 1513–1517. [Google Scholar] [CrossRef]
- Liu, W.J.; Xin, Z.C.; Xin, H.; Yuan, Y.M.; Tian, L.; Guo, Y.L. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J. Androl. 2005, 7, 381–388. [Google Scholar] [CrossRef]
- Ning, H.; Xin, Z.C.; Lin, G.; Banie, L.; Lue, T.F.; Lin, C.S. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology 2006, 68, 1350–1354. [Google Scholar] [CrossRef]
- Pan, Y.; Kong, L.D.; Li, Y.C.; Xia, X.; Kung, H.F.; Jiang, F.X. Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacol. Biochem. Behav. 2007, 87, 130–140. [Google Scholar] [CrossRef]
- Cheng, K.; Ge, B.F.; Zhen, P.; Chen, K.M.; Ma, X.N.; Zhou, J.; Song, P.; Ma, H.P. Effects of icariin and genistein on peak bone mass in rats. Acta Acad. Med. Sin. 2013, 35, 542–546. [Google Scholar]
- Zhang, Z.B.; Yang, Q.T. The testosterone mimetic properties of icariin. Asian J. Androl. 2006, 8, 601–605. [Google Scholar] [CrossRef]
- Xiong, Y.B.; Zhou, C.H. The effect of extracts from Herba Epimedii and Semen Cuscutae on the function of male reproduction. Chin. Pharm. J. 1994, 29, 89–91. [Google Scholar]
- Coonce, M.M.; Rabideau, A.C.; McGee, S.; Smith, K.; Narayan, P. Impact of a constitutively active luteinizing hormone receptor on testicular gene expression and postnatal Leydig cell development. Mol. Cell. Endocrinol. 2009, 298, 33–41. [Google Scholar] [CrossRef]
- Shiraishi, K.; Ascoli, M. Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology 2007, 148, 3214–3225. [Google Scholar]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Tang, P.Z.; Tsai-Morris, C.H.; Dufau, M.L. Regulation of 3beta-hydroxysteroid dehydrogenase in gonadotropin-induced steroidogenic desensitization of Leydig cells. Endocrinology 1998, 139, 4496–4505. [Google Scholar]
- Lecureuil, C.; Saleh, M.C.; Fontaine, I.; Baron, B.; Zakin, M.M.; Guillou, F. Transgenic mice as a model to study the regulation of human transferrin expression in Sertoli cells. Hum. Reprod. 2004, 19, 1300–1307. [Google Scholar] [CrossRef]
- Zakin, M.M. Regulation of transferrin gene expression. FASEB J. 1992, 6, 3253–3258. [Google Scholar]
- Sairam, M.R.; Krishnamurthy, H. The role of follicle-stimulating hormone in spermatogenesis: Lessons from knockout animal models. Arch. Med. Res. 2001, 32, 601–608. [Google Scholar] [CrossRef]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef]
- Johnston, H.; Baker, P.J.; Abel, M.; Charlton, H.M.; Jackson, G.; Fleming, L.; Kumar, T.R.; O'Shaughnessy, P.J. Regulation of sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 2004, 145, 318–329. [Google Scholar] [CrossRef]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Nat. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar]
- Hellani, A.; Ji, J.; Mauduit, C.; Deschildre, C.; Tabone, E.; Benahmed, M. Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 2000, 141, 3012–3019. [Google Scholar]
- Lui, W.Y.; Wong, C.H.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: An in vivo study. Endocrinology 2003, 144, 1139–1142. [Google Scholar] [CrossRef]
- Xia, W.; Wong, E.W.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009, 327, 48–61. [Google Scholar] [CrossRef]
- Nirupama, M.; Devaki, M.; Nirupama, R.; Yajurvedi, H.N. Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. J. Physiol. Biochem. 2013, 69, 59–68. [Google Scholar]
- Sze, S.C.; Tong, Y.; Ng, T.B.; Cheng, C.L.; Cheung, H.P. Herba Epimedii: Anti-oxidative properties and its medical implications. Molecules 2010, 15, 7861–7870. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Tang, Y.Z.; Liu, Z.Q. Protective effect of icariin on DNA against radical-induced oxidative damage. J. Pharm. Pharmacol. 2007, 59, 1729–1732. [Google Scholar] [CrossRef]
- Tamaki, R.; Yoshikawa, M.; Shinomiya, T.; Andoh, H.; Kawaguchi, M.; Hashimoto, A.; Byrne, D.W.; Kobayashi, H. Chronic administration of methamphetamine increases the mRNA expression of diazepam binding inhibitor in rat brain. Tokai J. Exp. Clin. Med. 2008, 33, 46–50. [Google Scholar]
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, A.; Liu, G.; Liu, H.; Wang, C.; Xia, T.; Chen, X.; Yang, K. Effects of p,p'-dichlorodiphenyldichloroethylene on the expressions of transferrin and androgen-binding protein in rat Sertoli cells. Environ. Res. 2006, 101, 334–339. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules 2014, 19, 9502-9514. https://doi.org/10.3390/molecules19079502
Chen M, Hao J, Yang Q, Li G. Effects of Icariin on Reproductive Functions in Male Rats. Molecules. 2014; 19(7):9502-9514. https://doi.org/10.3390/molecules19079502
Chicago/Turabian StyleChen, Maoxin, Jie Hao, Qiaozhen Yang, and Gang Li. 2014. "Effects of Icariin on Reproductive Functions in Male Rats" Molecules 19, no. 7: 9502-9514. https://doi.org/10.3390/molecules19079502




